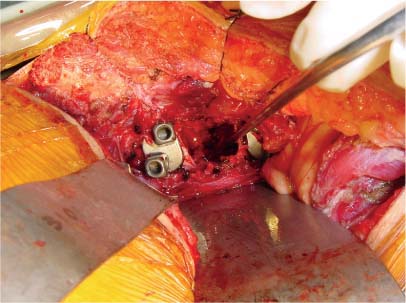
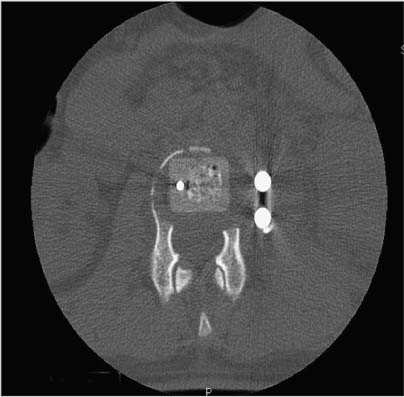
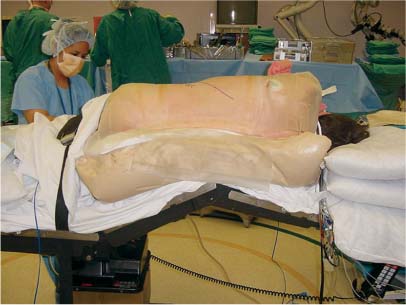
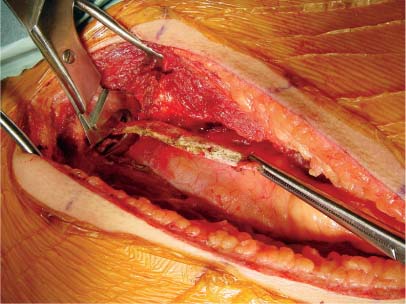
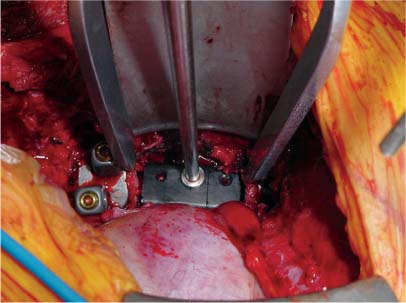
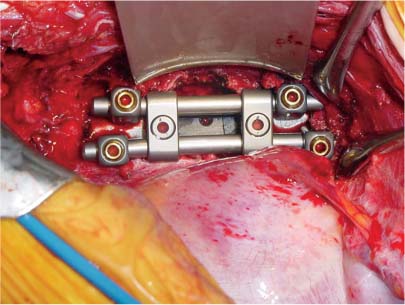
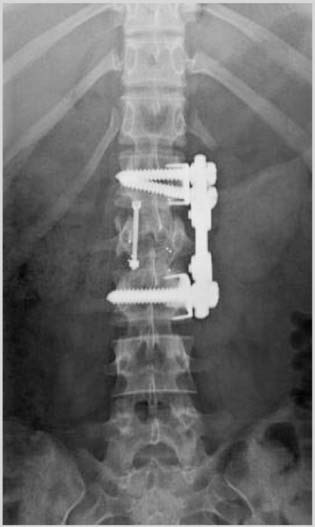
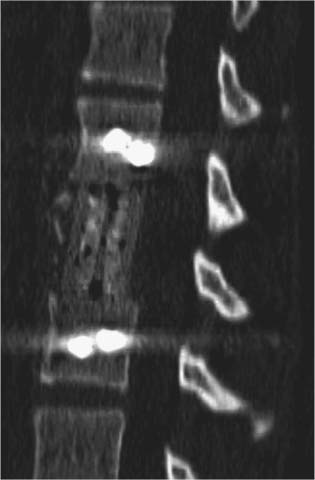
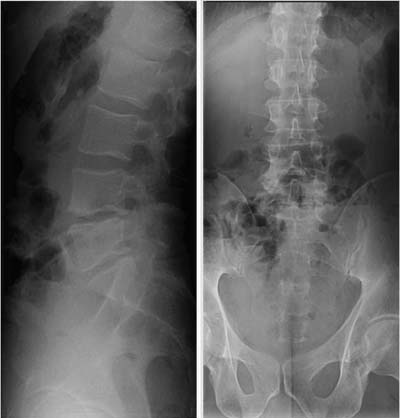
35 The location of the pathologic lesion and the goal of the surgical procedure best determine the surgical approaches to the lumbar spine and lumbosacral (LS) junction. In particular, approaches to the LS junction can be challenging because of its concavity; the posterior tilt of the sacrum below the sacral promontory; and the relationship of the LS junction to the pelvis, the iliac wings, and the overhang of the superior iliac crest. Combined anteroposterior (AP) approaches are often needed to adequately address pathology in this area of the spinal column. This chapter describes anterior surgical approaches to pathologic lesions involving the lumbar spine and LS junction. Anterior transperitoneal and retroperitoneal approaches to the lumbar spine and LS junction have been used extensively in the surgical treatment of degenerative disc disease, spinal deformities, vertebral osteomyelitis, traumatic burst fractures, fracture-dislocations, and tumors of the vertebral bodies. They are best used for exposing anterior pathology of the vertebral bodies, intervertebral discs, spinal cord, and nerve roots from L2 to the sacrum.1–6 They offer excellent anterior exposure of the thecal sac, and extensive bilateral anterior decompression and vertebral body resection can be performed via these approaches. Multiple vertebral body levels can be exposed using these approaches, and direct reconstruction of the anterior load-bearing support structures and stabilization with anterior instrumentation can be accomplished. The thoracolumbar junction is best exposed using the transthoracic approach.5,7,8 This is accomplished by detaching the diaphragm at its lateral periphery, allowing full exposure of the lower thoracic and upper lumbar vertebrae. The midlumbar spine (L2–L4) is best exposed using the retroperitoneal flank approach, and the lower lumbar spine and LS junction are best approached using a direct anterior approach via either a transperitoneal or an extraperitoneal approach.4,9–13 The principal disadvantage of anterior approaches is that they may frequently require a second procedure for decompression of posterior pathology or placement of posterior spinal instrumentation. The use of a left-sided approach for lower thoracic and thoracolumbar lesions (T7–L2) is advocated because it is technically easier to mobilize the aorta versus mobilizing the relatively friable vena cava or azygos venous system, and liver retraction is avoided.14 However, within these generalizations, the side of approach chosen should allow maximum exposure of the pathology being treated. After induction of anesthesia, the patient is positioned in a full lateral decubitus position with the side to be operated on in the “up” position. This places the spine in a 90-degree orientation to the floor, allowing for safer decompression and screw placement during instrumentation. The lower leg is kept straight and the upper leg is flexed, facilitating muscle retraction by relaxing the ipsilateral iliopsoas muscle. An axillary roll is placed beneath the axilla to prevent brachial plexus injury, and a pillow is placed between the legs. The level of interest is placed over the break in the operating table, which is flexed, to spread the rib cage and facilitate exposure. All pressure points are padded and the patient’s position is maintained using a beanbag or a “universal positioner.” The patient is then secured to the operating table using heavy tape. Fluoroscopy or plain x-rays are used to mark the level of interest and plan the incision. The surgeon may stand facing either the anterior15,16 or the posterior side of the patient, depending on his or her preference, and the assistant is situated on the opposite side. After the patient is prepped and draped in a sterile fashion, an incision is made over the 10th or 11th ribs for exposure of the thoracolumbar junction. The incision is carried obliquely and more ventrally depending on the extent of the caudal exposure required. The incision is carried down to the fascia, using the electrocautery. The latissimus dorsi and the serratus anterior and serratus posterior muscles are then identified and divided in line with the incision using the electrocautery. The appropriate level is confirmed using fluoroscopy or plain films, and a subperiosteal dissection is performed, exposing the 10th or 11th rib, with care taken not to violate the neurovascular bundle underlying the inferior aspect of the rib. The rib is resected, a padded Finochietto retractor is placed, and the ribs are spread. The chest is entered through the rib bed periosteum, the underlying endothoracic chest wall fascia, and the parietal pleura. The lung is retracted and packed with laparotomy pads, and the spine is observed through the parietal pleura.7,8,14 Single lung ventilation is not necessary at the thoracolumbar junction. After the chest is entered, the costal cartilage of the rib can be split longitudinally, allowing access to the properitoneal fat layer that lies caudal to the diaphragm and is continuous with the retroperitoneal space. The peritoneum is then cleared from the undersurface of the diaphragm, avoiding entry into the peritoneal cavity. The diaphragm is kept under tension and a circumferential incision is made in the medial portion of the diaphragm along its peripheral attachment to the costal margin, leaving a generous cuff for later reapproximation of the diaphragm. This allows exposure of the rostral retroperitoneal space. The spleen, kidneys, and stomach are gently retracted medially and caudally using a combination of gravity and a broad malleable retractor. The crus of the diaphragm is identified and cut at its attachment to the anterior longitudinal ligament (at approximately the L1–L2 level). The anterior surfaces of the vertebral bodies of the thoracolumbar junction are then visualized. After the appropriate vertebral body level is confirmed using fluoroscopy or plain x-ray, the segmental vessels are identified and ligated at the midpoint of the vertebral body. Care is taken to preserve the anastomotic vascular arcade in the region of the proximal neuralforamen to avoid ischemic spinal cord complications. This is best accomplished by ligating the segmental vessels on the side closer to the aorta. The disc spaces are identified and the appropriate decompression, reconstruction, and stabilization are performed depending on the goals of the operation. After the operation is completed, great care must be taken to accurately reattach the diaphragm to its costal margin. Nonabsorbable sutures are used for the diaphragmatic repair. The crus should be reattached to the anterior longitudinal ligament, and any tears in the peritoneum should be repaired. If possible, the parietal pleura overlying the vertebral column and any hardware is closed. A 28-French chest tube is placed and tunneled out through a separate incision inferior to the thoracotomy incision. The ribs are compressed and reapproximated with heavy absorbable suture. The rib bed is tightly reapproximated to reestablish a pleural seal. The intercostal musculature is closed using a running suture. The serratus anterior and the superficial muscle layers are also closed with a running suture. The subcutaneous tissue is closed with simple interrupted suture, and the skin is closed using a subcuticular suture or skin staples. The chest tube is secured to the skin in a standard fashion and placed to pleurovac suction. Anteroposterior and lateral plain film radiographs are obtained upon arrival at the recovery room or the intensive care unit. The chest tube is removed when drainage is less than 50 cc per 8 hours. A chest x-ray should be obtained 6 hours after the chest tube removal to rule out a delayed pneumothorax. Pulmonary complications are most frequent with transthoracic approaches. Atelectasis, pneumonia, pleural effusion, and chest wall discomfort are all possible with this approach. Rapid mobilization and early postoperative involvement of physiatrists and physical therapists are desirable for these patients. They prevent pulmonary complications and diminish the risks of postoperative thromboembolic complications. An appreciable number of patients (~10%) undergoing thoracotomy may experience prolonged chest wall pain postoperatively.17,18 An 18-year-old woman had fallen from a height of 20 feet. She complained of back pain but was neurologically intact, with no bowel or bladder symptoms. The patient’s surgery is described in Figs. 35-1 to 35-10. FIGURE 35-1 Plain film lateral radiograph demonstrates L2 fracture with loss of height and no significant sagittal plane alignment abnormalities. FIGURE 35-2 Axial computed tomography (CT) scan at the L2 pedicle level shows retropulsion of a fracture fragment between the pedicles occupying slightly more than half of the spinal canal (dotted line indicates the normal spinal canal). Follow-up: Two years after surgery, this patient remained neurologically intact postoperatively with normal bowel and bladder function. She has resumed all activities, including sports, with no restrictions. The retroperitoneal approach may be used for debridement, resection, and reconstruction of anterior lumbar vertebral pathology at L2–L5. First popularized by Harmon in 1950 as a treatment for degenerative disc disease, it is accomplished through an extension of the standard flank incision commonly used by general surgeons.1,4,19 It provides broad exposure of the lower lumbar vertebrae and upper sacrum with less risk to the viscera and great vessels when compared with the transperitoneal route; however, a relative disadvantage is that the vertebral bodies and intervertebral discs are viewed obliquely from one side. If a right-sided approach is necessary, the size and location of the liver can hamper exposure.16 FIGURE 35-3 Operative photograph demonstrates positioning for surgery: right lateral decubitus position with the left side up and the table maximally flexed at the L2 level. This degree of table flexion makes the approach easier and facilitates compression of the anterior strut later in the procedure (when the table is returned to a neutral position). FIGURE 35-4 Operative photograph of resection of the left 12th rib during the thoracoabdominal approach. Resecting this nonfunctional rib makes placement of spinal instrumentation at the L1 level easier. FIGURE 35-5 Operative photograph following L2 corpectomy with screws and plates above and below for later stabilization. The goal of the corpectomy is a pedicle-to-pedicle decompression with excision of the disc above and below. FIGURE 35-6 Operative photograph of placement of an anterior strut. In this case, the strut chosen was a stackable carbon fiber cage that was filled with local autologous bone obtained from the L2 vertebral body and the left 12th rib. With the strut held in the correct position, the table is returned to the neutral (unflexed) position under direct observation. Straightening the operating room table compresses the strut between the adjacent vertebrae. FIGURE 35-7 Operative photograph of the final construct after the rods have been applied between the vertebral body screws. This is performed after the table has been returned to the neutral position. Cross-connectors are placed between the rods to improve rotational and torsional stability. FIGURE 35-8 Plain film anteroposterior (AP) radiograph at 6 months postoperatively. The left 12th rib was resected. The screws above and below the corpectomy are placed bicortically and the spine is well aligned in the coronal plane. FIGURE 35-9 Axial CT scan demonstrates a thin rim of cortical bone left anteriorly and on the contralateral side from the approach. A pedicle-to-pedicle decompression was achieved, and the strut is well centered with respect to the adjacent vertebrae. The patient is positioned on the operating table in the lateral decubitus position with the side to be operated on tilted upward, at an angle between 30 and 60 degrees. The shoulder and hips are supported on bean-bags. This shifts the abdominal and retroperitoneal contents toward the nonoperated, dependent side. The arm on the operated side is held across the chest and supported on a Krause splint. The lower leg is kept straight and the upper leg and hip are flexed to facilitate muscle retraction by relaxing the ipsilateral iliopsoas muscle. An axillary roll is placed beneath the axilla, and a pillow is placed between the legs. The surgeon stands posterior to the patient, with the assistant standing anterior. All pressure points are padded, and the patient is secured to the operating table using heavy tape. Fluoroscopy or plain x-rays are used to mark the level and plan the incision. The spine is usually approached from the left side unless the pathology dictates a right-sided approach. As mentioned previously, the liver limits right-sided approaches, and the thin-walled vena cava is located on the right side. Hemorrhage resulting from vena caval injury can be difficult to control. From a left-sided approach, the smaller spleen is easier to retract, and the thick-walled pulsatile aorta is easily located and therefore is less susceptible to injury.20 FIGURE 35-10 Sagittal CT scan reconstruction reveals good positioning of the strut with maintenance of normal sagittal plane alignment. After the patient is prepped and draped in a sterile fashion, a transverse flank incision is made equidistant between the lowest rib and superior iliac crest in the midaxillary line and extended medially to the lateral edge of the rectus sheath. The placement of the incisions varies according to the spinal level approached. The external oblique fascia is identified and the muscle is opened in line with the incision to the lateral border of the rectus fascia. The internal oblique and the transversus abdominis muscles are also opened in line with the skin incision. The transversalis fascia is identified and opened in the posterior portion of the wound with blunt scissors, and the retroperitoneal space is entered. The peritoneum is recognized as a thin, translucent membrane and is dissected free from the transversalis fascia medially. The abdominal wall incision is extended medially, after retracting the peritoneum. Dissection of the medial muscle layers and fascia is more difficult, and peritoneal violation can occur. Tears in the peritoneum should be immediately repaired with absorbable suture using an atraumatic needle. Dissection proceeds along the peritoneum and renal fascia posterior to the kidney between the renal fascia and the quadratus lumborum and psoas muscles. There is a natural cleavage plane that it is easily developed with blunt dissection immediately posterior to the renal fascia. Avoid dissection into the fat posterior to the renal fascia; this leads to a blind space behind the psoas muscle. Mobilize the peritoneum medially off the posterior abdominal wall from the inferior pole of the left kidney to the sacrum. This exposes the anterior surfaces of the quadratus lumborum and psoas muscles. The sympathetic chain and genitofemoral nerve, a small white structure lying on the psoas muscle, are identified. The ureter is identified by its cylindrical shape, peristaltic movements, and rich superficial vascular network, and it is mobilized medially to avoid injury.21,22 A padded Bookwalter or OMNI self-retaining retractor is used to open the wound and to retract the kidney and the peritoneal contents medially.23 The lumbar vertebral bodies are easily palpated, and the anterolateral aspects of the L2–L5 vertebral bodies are easily visualized. As in the thoracic spine, the vertebral bodies are relatively concave, and the intervertebral discs are convex. After the level is confirmed using fluoroscopy or radiographs, the psoas muscle is mobilized posteriorly, as necessary, using a Cobb elevator. Particularly in the lower lumbar spine, care should be taken to avoid avulsing psoas muscle fibers, which can lead to postoperative hip flexor weakness. The sympathetic chain is preserved as much as possible by gentle blunt dissection, and bipolar electrocautery is used instead of unipolar. The segmental vessels are isolated, ligated, and transected midway between the parent vessel and the neural foramen. This minimizes the risk of compromising the arterial blood supply to the neural foramen.4 After ligating the segmental vessels, the anterior surface of the vertebral body is exposed by bluntly dissecting between the anterior longitudinal ligament and the vertebral body with a Cobb elevator. The disc spaces are identified and the appropriate decompression, reconstruction, and stabilization are performed depending on the goals of the operation. If upper lumbar (L1–L2) exposure is needed, the skin incision is curved proximally to the end of the 11th or 12th rib. After entering the retroperitoneum, upper lumbar exposure is achieved by retracting the lower pole of the kidney medially and superiorly. After mobilizing the ureter, the left diaphragmatic crus, which extends to the second lumbar vertebral body, can be taken down. Exposure of the lower lumbar spine is most easily performed with minimal or no tilt to the operating table. At the L5–S1 level, the disc space and vertebral bodies are usually exposed between the common iliac arteries. At this level, a flat orientation to the operating table allows optimal visualization and orientation to the anatomic midline. The true anatomic midline should be confirmed fluoroscopically with an image that bisects the pedicles. Most surgical difficulties in this region result from a loss of identification of the midline, which can lead to lateral dissections and suboptimal placement of instrumentation. The disc can be palpated by reaching over the left common iliac artery in the bifurcation. The dissection is carried down to the anterior longitudinal ligament, which is medial to the left common iliac vessels. The prevertebral tissues, which include the hypogastric plexus and middle sacral vessels, are swept, en bloc, to the right side. No monopolar electrocautery is used from this point in the operation, as it is associated with injury to the sympathetic plexus and resultant retrograde ejaculation. The iliac vessels are retracted laterally by inserting Steinmann pins into the vertebral bodies. If the vascular anatomy prevents interiliac exposure, the left common iliac vessels can be reflected to the right side, and the interspace exposed from the left. Prior to mobilizing the left iliac vein, the iliolumbar vein must be identified and ligated. This vessel is usually located 2 to 3 cm distal to the bifurcation of the iliac vessels. For extensive LS junction exposure, both the common iliac artery and vein can be completely mobilized. This allows the spine to be approached either medially or laterally to the iliac vessels.24 Wound closure is straightforward. All peritoneal violations are repaired, and each layer of the abdominal wall is identified and carefully reapproximated. The superficial soft tissues are closed in a standard multilayered anatomic fashion. The transperitoneal approach to the lumbar spine was first described for the treatment of spondylolisthesis. In the 1940s and 1950s, it was used to perform lumbar interbody fusions as a treatment for degenerative disc disease.10,25 Transperitoneal exposures provide excellent visualization of the lower lumbar vertebral bodies, with optimal exposure of the L5–S1 interspace. Although the abdominal viscera can be easily retracted away from the spine, the great vessels and the hypogastric nerve plexus must be carefully mobilized before exposing the spine. This increases the risks of vascular complications and can cause impotence in males.20,26,27 Collaboration with a general or vascular surgeon may decrease the frequency of these complications. Preoperative preparation includes a bowel prep the day before surgery. The patient is placed supine with the sacral region elevated by angling the operating table under the sacral region between 0 and 40 degrees depending on the vertebral segment exposed. The patient is prepped and draped to expose the abdominal operative field and the bone graft site if necessary. A midline, para-midline, or transverse (Pfannenstiel) incision may be used with fluoroscopy to identify the correct level of the spinal lesion. The abdominal wall fascia is incised in the midline and the peritoneum opened, with care taken to avoid bowel injury. The Trendelenburg position is used to maintain the intestines in the upper portion of the abdomen. The redundant parts of the sigmoid, cecum, and small bowel are displaced into the upper portion of the abdomen and retracted with moist laparotomy sponges to expose the lower lumbar and pelvic portions of the posterior peritoneum. The pelvic portion of the colon and its mesentery are then retracted to the left, and the ureters are identified. After the bowel is mobilized, the aortic bifurcation and sacral promontory are seen through the posterior peritoneum. The prevertebral neurovascular structures include the aorta, vena cava, their collateral vessels, and the hypogastric nerve plexus. The anatomy of these structures is variable, and knowledge of possible variations is necessary to safely expose the anterior LS spine.4,12 The transperitoneal approach is best used for exposure of the L5–S1 interspace. This disc space is usually located several centimeters below the aortic bifurcation and can be palpated, and readily identified, between the common iliac vessels. The peritoneum is grasped with a toothless forceps above the aortic bifurcation and opened in the midline with blunt scissors. The incision is continued caudally following the right common iliac artery to its bifurcation at the external and internal iliac arteries. The right ureter is identified and avoided as it crosses under the right external iliac artery. The peritoneal flaps are freed and reflected laterally to the common iliac arteries. The hypogastric plexus is located directly anterior to the fifth lumbar vertebral body and the L5–S1 disc space. The dissection is continued down to the anterior longitudinal ligament just medial to the left iliac artery and vein to avoid injuring the hypogastric plexus. The prevertebral tissues, including the hypogastric plexus, are then swept from right to left by bluntly dissecting directly on the anterior surface of the disc. By displacing these structures en bloc and neither dissecting through nor cauterizing them, there is less risk of injuring the hypogastric plexus. The middle sacral artery is sometimes adherent to the vertebral bodies, and bleeding may occur when mobilizing it. Hemorrhage can often be controlled with packing and pressure. Use of bipolar electrocautery or vascular clips instead of the monopolar electrocautery reduces the risk of hypogastric plexus injury. The iliac vessels are laterally retracted to expose the L5–S1 disc space by placing four Steinmann pins with rubber sleeves into the vertebral bodies under direct visualization. When a large left common iliac vein hinders access to the body of L5 and the sacral promontory, the vein should be dissected free from the left common iliac artery. Place vessel loops proximally and distally around the vein with a right angle dissector and retract the vein. This collapses the vein, allowing mobilization and access to the vertebral bodies. The left iliac vein can occasionally appear as a flat, white, bloodless ribbon across the L5–S1 disc space. Brisk hemorrhage occurs if this vessel is not properly identified and mobilized before incising the disc. After appropriate decompression and reconstruction, the posterior parietal peritoneum is closed using 3–0 Vicryl suture. Drainage is not required. Anatomically repositioning the bowel loops prevents bowel torsion, and places the greater omentum over the intestines. The abdominal fascia and anterior parietal peritoneum are closed with interrupted sutures. The muscle, subcutaneous tissue, and skin are closed as separate anatomic layers. There are several advantages of the anterior extraperitoneal approach over a flank approach. It offers direct extensive bilateral anterior exposure to the vertebral bodies from L2 to the sacrum, avoiding division of the lateral abdominal muscles and the need to contend with the psoas muscle. Its principal disadvantage is the extensive manipulation of the great vessels required to gain exposure and the higher incidence of male sexual dysfunction associated with it as compared with flank incisions.18 Preoperative preparation includes a bowel prep the day before surgery.16 The patient is placed supine with the lower lumbar spine at the level of the kidney rests. Angling the operating table under the sacral region between 0 and 40 degrees, depending on the vertebral segment exposed, elevates the sacral region. The patient is prepped and draped to expose the abdominal operative field and the bone graft site if necessary. The spine is usually approached from the left side unless the pathology dictates a right-sided approach for the same reasons outlined previously. The lateral edge of the left rectus abdominus muscle is identified by palpation ~5 cm lateral to the midline. A midline, para-midline, or lower abdominal pararectus incision may be used with fluoroscopy to identify the level of the spinal lesion.5,9,11 The incision is deepened through the subcutaneous fat to the abdominal wall fascia using electrocautery. Hemostasis is obtained, self-retaining retractors are placed, and the lateral border of the rectus muscle is again palpated and identified. An incision is then made in the anterior rectus sheath along the extreme lateral edge of the rectus muscle. The fibers of the rectus muscle are slightly retracted medially to visualize the posterior rectus fascia and the arcuate line. An attempt should be made to preserve the epigastric vessels during this dissection, but if they are damaged, they can be safely ligated without any adverse sequelae. The transversalis fascia should be identified. The transversalis fascia is the internal investing fascial layer of the abdominal cavity. Above the arcuate line, the abdominal wall deep to the rectus muscle consists of the posterior rectus fascia and transversalis fascia. Inferior to the arcuate line, the abdominal wall consists only of the transversalis fascia. The most direct way of entering the preperitoneal space is to incise the fibers of the arcuate line and the underlying transversalis fascia. The outer surface of this transversalis fascia is dissected to the edge of the rectus sheath. At this point, the transversalis fascia is dissected splits to form the lamina of the rectus sheath. The transversalis fascia is incised lateral to the linea semilunaris with care taken to avoid entering the underlying peritoneal cavity. The incision begins in line with the skin incision. The peritoneum is bluntly dissected medially with a sponge off the undersurface of the transversalis fascia. This should be carried superiorly to develop a plane under the posterior rectus fascia. After this plane is developed, the peritoneum is retracted and the posterior rectus and transversalis fascia are divided along the lateral edge of the rectus muscle, thus exposing the peritoneum throughout the entire length of the incision. The peritoneum is swept medially in a left to right direction using blunt dissection to allow for exposure of the left iliac fossa. The psoas muscle is identified and the peritoneum is disseceted bluntly, off the psoas toward the midline. The genitofemoral nerve should be identified on the anterior aspect of the psoas muscle. Next, the ureter, aorta, and left iliac vessels are identified. The peritoneum should be mobilized medially, with the ureter, off the posterior abdominal wall from the inferior pole of left kidney to the sacrum. This exposes the anterior surfaces of the quadratus lumborum and psoas muscles and the sympathetic chain and genitofemoral nerve—a small white structure lying on the psoas muscle. The ureter is identified by its cylindrical shape, peristaltic movements, and rich superficial vascular network, and mobilized medially to avoid injury. A padded Bookwalter or OMNI self-retaining retractor is used to open the wound and to retract the kidney, ureter, the great vessels, and the peritoneal contents medially. Care should be taken to avoid excessive traction on the ureter and great vessels. The lumbar spine can be palpated deep to the medial edge of the psoas muscle at this point. The hills and valleys represent the disc spaces and the vertebral bodies, respectively. After the level is confirmed using fluoroscopy or radiographs, attention is turned to fully exposing the levels of interest. Initial exposure of the lumbar spine is accomplished by dissecting the medial edge of the psoas muscle laterally off the vertebral bodies using a Cobb elevator. As the vertebral bodies are exposed, the left segmental arteries and veins are identified at the midpoint of the vertebral bodies and individually ligated, if necessary, close to their origin at the aorta. This minimizes the risk of compromising arterial blood supply to the neural foramen. After ligation of the segmental vessels, the vertebral bodies are visualized, and a plane can be developed between the vertebral bodies and the anterior longitudinal ligament, thus exposing the entire anterior surface of the vertebral body. This dissection can be continued superiorly to L2 and inferiorly to the sacrum, depending on the extent of exposure required.9,28 If upper lumbar exposure is needed, the skin incision can be carried superiorly to the inferior aspect of the 10th, 11th, or 12th ribs. After entering the retroperitoneum, the upper lumbar exposure is facilitated by rotating the kidney superiorly and medially. Occasionally, a lumbar vein arises from the posterior aspect of the renal vein. This vessel must be ligated before the kidney can be retracted. Failure to identify and ligate this vessel can lead to vessel avulsion and troublesome bleeding. Distal lumbar spine and LS exposure demands caution because the left iliac vessels and their branches, which overlie the lower lumbar spine and sacrum, are fragile. These vessels must be mobilized to adequately expose the lower lumbar spine and LS junction. The common iliac artery is usually free of branches and can be easily mobilized down to the iliac bifurcation. As previously stated, the left iliolumbar vein should be ligated prior to mobilizing the left common iliac vein. The superior hypogastric plexus, located anterior to the origin of the left iliac artery, should be preserved in males to avoid sexual dysfunction and retrograde ejaculation with resultant sterility.26,27 Exposure of the LS junction and the sacrum can usually be accomplished without mobilization of the iliac vessels by ligating the middle sacral artery; however, for extensive exposure from L2 to the LS junction, the bifurcation of both the common iliac artery and vein located at the L4–L5 interspace must be completely mobilized to allow full exposure of the lumbar spine and the LS junction. This allows LS exposure either medial or lateral to the iliac vessels. Iliac artery bifurcation mobilization requires ligation of several branches of the internal iliac artery, including the iliolumbar and lateral sacral arteries. These vessels are ligated and divided close to their origins. If necessary, the main internal iliac artery trunk can be unilaterally ligated and divided, but individual branch ligation is preferred.9,24,29 Internal iliac vein mobilization is challenging because of its numerous tributaries. Dissection and division of the investing fascia off of the common iliac vein usually facilitate mobilization and identifies the origins of the external and internal iliac veins. Occasionally, there may be lumbar veins that empty into the external iliac vein, but the internal iliac vein receives the majority of the pelvic venous flow; therefore, internal iliac vein ligation should be avoided because it increases pelvic venous pressure and may promote venous bleeding, which can be difficult to control. Exposure of the superior sacrum requires careful elevation of the internal iliac vein off the lateral aspect of the sacrum. The presacral fascia covers the branches of the internal iliac vein. This fascia firmly tethers the internal iliac vein in the pelvis and must be incised for safe mobilization of the vein. The ilio-lumbar, lateral sacral, and presacral venous tributaries must be ligated as they enter the internal iliac vein to mobilize the internal iliac vein. Due to the proximity of the iliac vessels to the anterior lumbar spine near the iliac bifurcation (L4–L5), placement of surgical hardware can be challenging. If the vessels will directly contact metallic hardware, it may be preferable to place a cage of bone graft to reconstruct the anterior column of the spine and forgo placing hardware. In this scenario, a posterior stabilization procedure may be necessary to stabilize the lower lumbar spine or the LS junction. After appropriate decompression and reconstruction, wound closure is straightforward. Anatomically, the peritoneum is repositioned, and all peritoneal violations are identified and repaired. Each layer of the abdominal wall are carefully identified and repaired. The superficial soft tissues are closed in a standard multilayered anatomic fashion. Vascular, bowel, peripheral nerve, and ureteral injuries are possible complications with the retroperitoneal, extraperitoneal, and transperitoneal LS approaches.18,21,30,31 Furthermore, because one or more of the major anterior abdominal wall muscles are divided, wound dehiscence, herniation, and hematoma can also occur. Vascular and bowel injuries are more likely to occur with transabdominal approaches, whereas peripheral nerve and ureteral injuries occur more commonly with the retroperitoneal and extraperitoneal approaches. Both approaches are associated with sterility in males secondary to retrograde ejaculation caused by damage to the superior hypogastric plexus.26,27 The superior hypogastric plexus is located directly anterior to the fifth lumbar vertebral body and the L5–S1 disc space and carries sympathetic autonomic fibers. During ejaculation, it causes the smooth muscle of the seminal vesicles to contract as the bladder neck closes. It is responsible for sperm transport from the testes to the seminal vesicles via the vas deferens. Thus, damage to the superior hypogastric plexus can result in sterility through loss of normal sperm transport or secondary to retrograde ejaculation caused by failure of seminal vesicle contraction during ejaculation. There is a variable reported incidence of retrograde ejaculation with anterior approaches to the LS junction. Impotence can also occur if the parasympathetic fibers responsible for penile erection, arising from the S2–S4 segments and carried in the inferior hypogastric plexus, are damaged during the resection of extensive sacral lesions.22,26,27 The surgeon should be prepared for vascular anomalies, and vascular instruments should always be available when performing retroperitoneal or transperitoneal approaches. If injury to one of the great vessels occurs, compression above and below the injury or placing temporary vascular clamps will reduce the hemorrhage while the defect is being repaired.20,24,31 Excessive traction on diseased calcified arteries, often found in elderly patients, is avoided to prevent fracture of an atherosclerotic plaque and possible arterial embolization or thrombosis. In addition, because venous structures are mobilized, the incidence of postoperative deep venous thrombosis is significant, and consideration should be given to perioperative prophylactic anticoagulation.18,32 Care must be taken throughout the procedure to avoid compressing the iliac arteries or veins for prolonged periods to further avoid vessel thrombosis. As a result, we ordinarily employ self-retaining retractors on the viscera and utilize hand-held retractors on the vessel whenever possible. Ureteral injury or delayed ureteral fibrosis may occur from manipulation or traction. For this reason, if ureteral mobilization is necessary, a generous portion of the periureteric tissue is included to avoid compromising ureteral blood supply.21 Perforation of the peritoneum during retroperitoneal approaches is not uncommon, and peritoneal tears should be immediately repaired. All bowel perforations must be immediately irrigated and oversewn, usually with the help of a general surgeon.16,31 Accurate meticulous closure of all traversed abdominal wall muscle layers must also be accomplished to avoid wound dehiscence, hematomas, and abdominal incisional hernias.33,34 A 42-year-old man, a kitchen cabinet installer, developed severe mechanical lower back pain. He showed no lasting response to a prolonged conservative treatment regimen, including physical therapy with emphasis on core strengthening and epidural injections. Imaging studies were obtained when it was clear that the conservative treatment plan was not succeeding. The patient’s surgery is described in Figs. 35-11 to 35-19. Follow-up: The patient had an uneventful postoperative course and has resumed an activity level that closely compares to his preoperative status. He has been weaned down from the significant preoperative narcotic medication dosage to minimal medication on an “as needed” basis. He remains neurologically intact with no bowel, bladder, or sexual dysfunction. He was able to resume working 4 weeks after surgery.
Anterior Transperitoneal and Retroperitoneal Approaches to the Lumbar Spine and Lumbosacral Junction
 Operative Techniques
Operative Techniques
The Thoracolumbar Junction and Upper Lumbar Spine
Complications
Illustrative Case 1
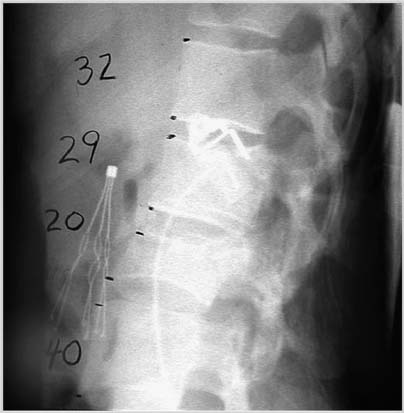
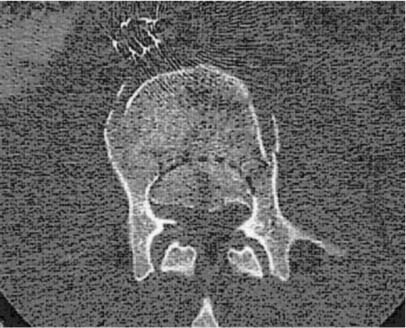
Retroperitoneal Flank Approach to the Lower Lumbar Spine and Lumbosacral Junction
Transperitoneal Approach
Extraperitoneal Exposure of the Lumbar Spine and Lumbosacral Junction
Complications
Illustrative Case 2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree