(Video 17.1). If any of these symptoms are present, a detailed characterization is performed. The clinical significance of hearing loss is related to the time and acuity of onset, severity, and the tendency to fluctuate or progress. The deleterious effects of hearing loss are particularly great when the onset occurs before the development of spoken language (i.e., prelingual hearing loss).
B. Physical examination.
1. The otologic examination begins with inspection of the pinna and palpation of periauricular structures, including the periauricular and parotid lymph nodes.
2. Otoscopic examination of the external ear canal and tympanic membranes is performed to identify abnormalities of these structures. Pneumatic otoscopy is helpful in assessing the mobility of the tympanic membrane and is particularly useful in identifying a subtle middle ear effusion.
3. A complete head and neck examination is performed, including a cranial nerve and cerebellar testing.
4. Tuning fork tests are an important part of the otologic functional examination for hearing acuity. They are particularly useful in differentiation between conductive and sensorineural hearing loss. The most useful tuning forks are those with vibrating frequencies of 512 and 1,024 cycles per second. The two most commonly used tuning fork tests are Weber’s test and Rinne’s test.
a. Weber’s test is performed by placing the stem of the tuning fork on the midline plane of the skull. The patient is asked to identify the location of the auditory percept within the head. The signal lateralizes to the ear with conductive hearing loss provided normal hearing is present in the opposite ear. This occurs because the ambient room noise present in the usual testing situation tends to mask the normal ear, but the poorer ear with a conductive loss does not hear such noise and better hears bone-conducted sound. If a sensorineural loss is present in one ear and the opposite ear is normal, the fork is heard louder in the better ear.
b. Rinne’s test is performed by alternately placing a ringing tuning fork opposite one external auditory meatus and firmly on the adjacent mastoid bone. The loudness of the tuning fork in these two locations is compared. The normal ear hears a tuning fork about twice as long with air conduction as with bone conduction. Conductive hearing loss reverses this ratio, and sound is heard longer with bone conduction than with air conduction. Patients with sensorineural hearing loss hear better by means of air conduction than by means of bone conduction, although hearing is reduced with both air and bone conduction.
AUDIOLOGIC EVALUATION
The audiologic evaluation characterizes the type, severity, and configuration of a hearing loss. Loss of hearing can be either partial or total. It can affect the low, middle, or high frequencies in any combination.
A. Range of hearing. Although the human ear is sensitive to frequencies between 20 and 20,000 Hz, the frequency range from 300 to 3,000 Hz is most important for understanding speech.
1. During an audiologic evaluation, pure-tone thresholds are routinely obtained for frequencies at octave intervals between 250 and 8,000 Hz.
2. The range of sound pressure to which the human ear responds is immense. Infinitesimal movement of the hair cells produces a just audible sound, yet a million-fold increase is still tolerable.
3. The large range of numbers needed to describe audible sound pressure is best represented by a logarithmic ratio comparing a sound to a standard reference sound. This is called the decibel. The decibel is defined in relation to the physical reference of sound, or sound pressure level, to the average threshold of normal hearing for young adults, or hearing level (HL), or to a patient’s own threshold for the sound stimulus, or sensation level.
4. Speech sounds vary in their acoustic characteristics. Vowels tend to have most of their energy in the low to middle frequencies and are produced at relatively higher intensities than consonants. Thus, vowels carry the power of speech. Consonants tend to contain higher-frequency information and have low power. Much of the actual understanding of speech depends on the correct perception of consonants. Consequently, speech may not be audible for patients with hearing loss across the entire frequency range. Patients with hearing loss in the higher frequencies may hear speech but not understand it.
B. Audiogram. To graphically represent the degree of hearing, pure-tone thresholds are displayed on an audiogram (Fig. 17.1). On this graph, frequency (pitch) is represented on the horizontal axis and intensity (loudness) is presented on the vertical axis. The 0 dB HL line represents the average threshold level for a group of normal-hearing young adults with no history of otologic disease or noise exposure. Conversational speech at a distance of 1 m has an intensity level of approximately 50 to 60 dB HL. Speech becomes uncomfortable to listen to at approximately 80 to 90 dB HL.
C. Pure-tone threshold audiometry. The audiometric threshold is defined as the softest intensity level of a pure-tone signal that can be detected by the patient 50% of the time. Thresholds are generally obtained for air-conduction stimuli presented through earphones or in a sound field, and for bone-conduction stimuli presented with a vibrator placed on the mastoid or forehead.
For adults and older children, pure-tone testing simply requires a behavioral response to pure-tone stimulation. For infants older than 5 months, visual reinforcement audiometry can be used to obtain thresholds. In this operant discrimination task (i.e., yes–no paradigm), infants are trained to turn to their right or left when they hear a signal, where they see an illuminated animated toy. Alternatively, play audiometry is used to assess the hearing of preschool children. In this technique, play activities are used as operant reinforcers for a child’s response to auditory signals.
The degree of hearing loss is classified in terms of audiometric thresholds and are slight (16 to 25 dB HL), mild (26 to 40 dB HL), moderate (41 to 55 dB HL), moderately severe (56 to 70 dB HL), severe (71 to 90 dB HL), and profound (>90 dB HL).
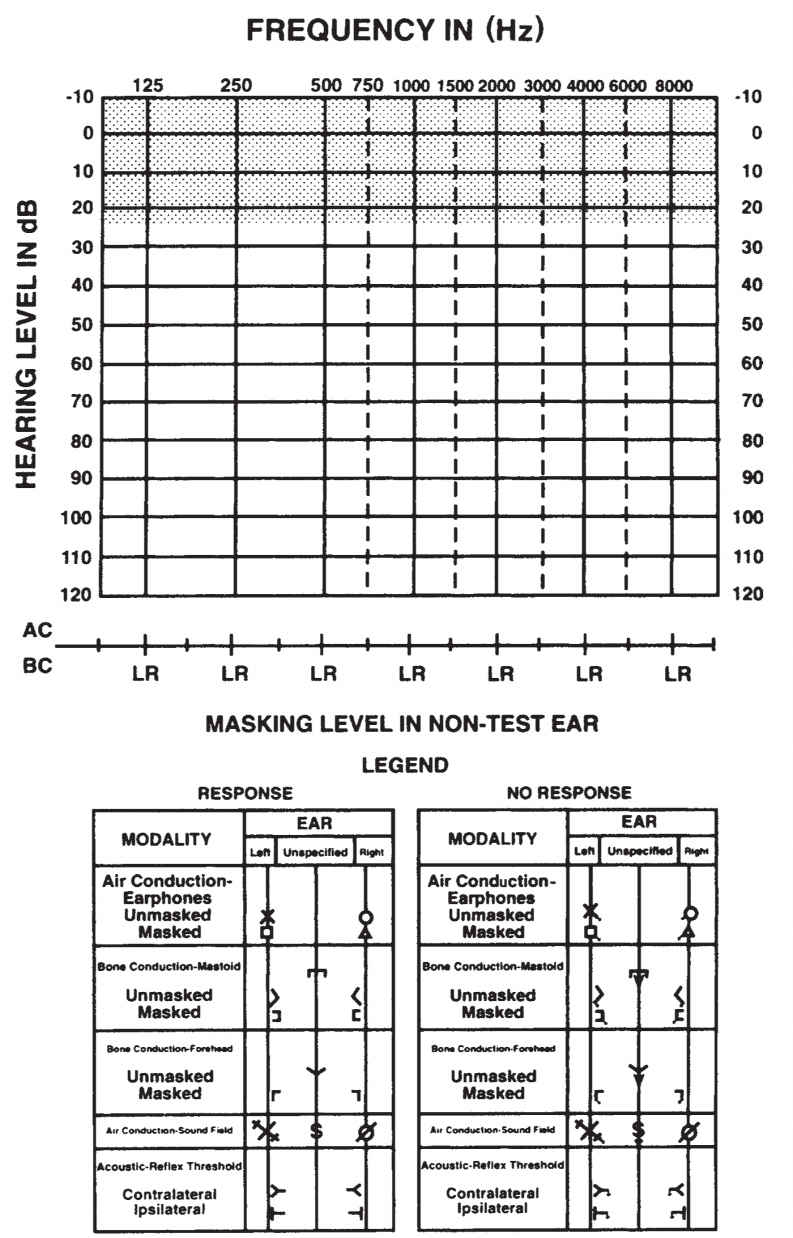
FIGURE 17.1 A sample audiogram. The y-axis represents the intensity level in dB HL and the x-axis represents the pure-tone frequency of the stimulus. The threshold is the softest level that the patient hears the pure-tone signal 50% of the time.
D. Speech audiometry. Speech signals can be used to assess hearing sensitivity and the processing capabilities of the auditory system. For some patients, speech can be audible but not easily understood because of various physiologic and environmental factors. The following tests are designed to assess both the audibility and intelligibility of speech.
1. Speech threshold tests. A speech recognition threshold (SRT) and a speech awareness threshold (SAT) are used to examine sensitivity to speech. These tests can be obtained for each ear individually or for both ears via sound field testing. The SRT is obtained at the lowest intensity level at which the patient can repeat spondee words (i.e., two-syllable words with equal stress on each syllable) 50% of the time. The SAT is the lowest intensity level that allows the patient to detect the presence of speech. The SRT and the SAT are used to provide a valid estimate of hearing sensitivity and to verify the accuracy and reliability of the pure-tone thresholds. The SRT and pure-tone average of the thresholds obtained at 500, 1,000, and 2,000 Hz should be within ±7 dB of one another. If a discrepancy exists, the examiner should doubt the validity or accuracy of the patient’s thresholds.
2. Speech discrimination. Word recognition or speech discrimination testing determines how well a patient can understand speech when the stimuli are presented at suprathreshold intensity levels. Speech recognition or discrimination scores depend on the type, severity, and configuration of the hearing loss and on the type of pathologic condition of the ear. The scores depend on a number of stimulus and response characteristics. The patient’s attending and cognitive skills also can influence the results, particularly in examinations of children and elderly persons. Although several pathologic conditions can markedly decrease speech recognition or discrimination scores, a rollover phenomenon in which the scores first increase and then dramatically decrease with increasing presentation levels is a characteristic of retrocochlear lesions.
E. Screening for hearing loss. Because hearing is critical for speech and oral language development in children, early identification of hearing loss is a primary concern for health care professionals and educators. The Joint Committee on Infant Hearing 2007 Position Statement and Guidelines endorse universal hearing screening of newborn infants before 1 month of age. For infants who fail their initial screening, it is recommended that a comprehensive audiologic evaluation be completed by 3 months of age. For infants with a hearing loss, appropriate health care and educational provisions should be made by 6 months of age. It is also recommended that regular audiologic and communication screenings be conducted within the first 3 years of life for all children.
PHYSIOLOGIC MEASURES OF HEARING
Whenever possible, behavioral measures of hearing should be used to assess the status of the auditory system. However, because of many variables that can affect the validity and reliability of these measures, particularly when testing the hearing of infants and young children, physiologic techniques can be used to assess the integrity of the auditory system.
A. Immittance audiometry is an objective means for determining the integrity of the middle and external ear cavities and can provide information about middle ear pressure, the mobility of the tympanic membrane, eustachian tube functioning, the mobility of the ossicles, and acoustic reflex thresholds (ARTs).
1. Tympanometry is used to assess disorders of the middle ear that affect the tympanic membrane, middle car space, and ossicular chain. It provides information about the mobility of the tympanic membrane in response to changes in air pressure presented to the external auditory canal. Tympanograms typically present the amount of compliance as a function of air pressure and are classified as Type A (normal middle ear function—see Fig. 17.2), Type B (flat—no change in compliance with change in external ear canal pressure), or Type C (negative middle ear pressure that may indicate the presence of fluid in the middle ear). This testing, however, can lack specificity for infants younger than 6 months because of the high compliance of their external ear canal walls.
2. ART. When an ear with normal hearing is exposed to an intense auditory signal, the stapedius muscle contracts. This contraction can be measured as changes in ear canal pressure. It can be elicited in normal-hearing individuals using pure-tone signals that vary between 70 and 100 dB HL. The lowest intensity level that produces this response is referred to as the ART. This acoustic reflex occurs bilaterally regardless of which ear is stimulated, if the system is functioning normally. The presence or absence of the reflex and the intensity levels at which the reflex is obtained provide information useful in identifying lesions within the auditory system up to the level of the superior olivary complex.
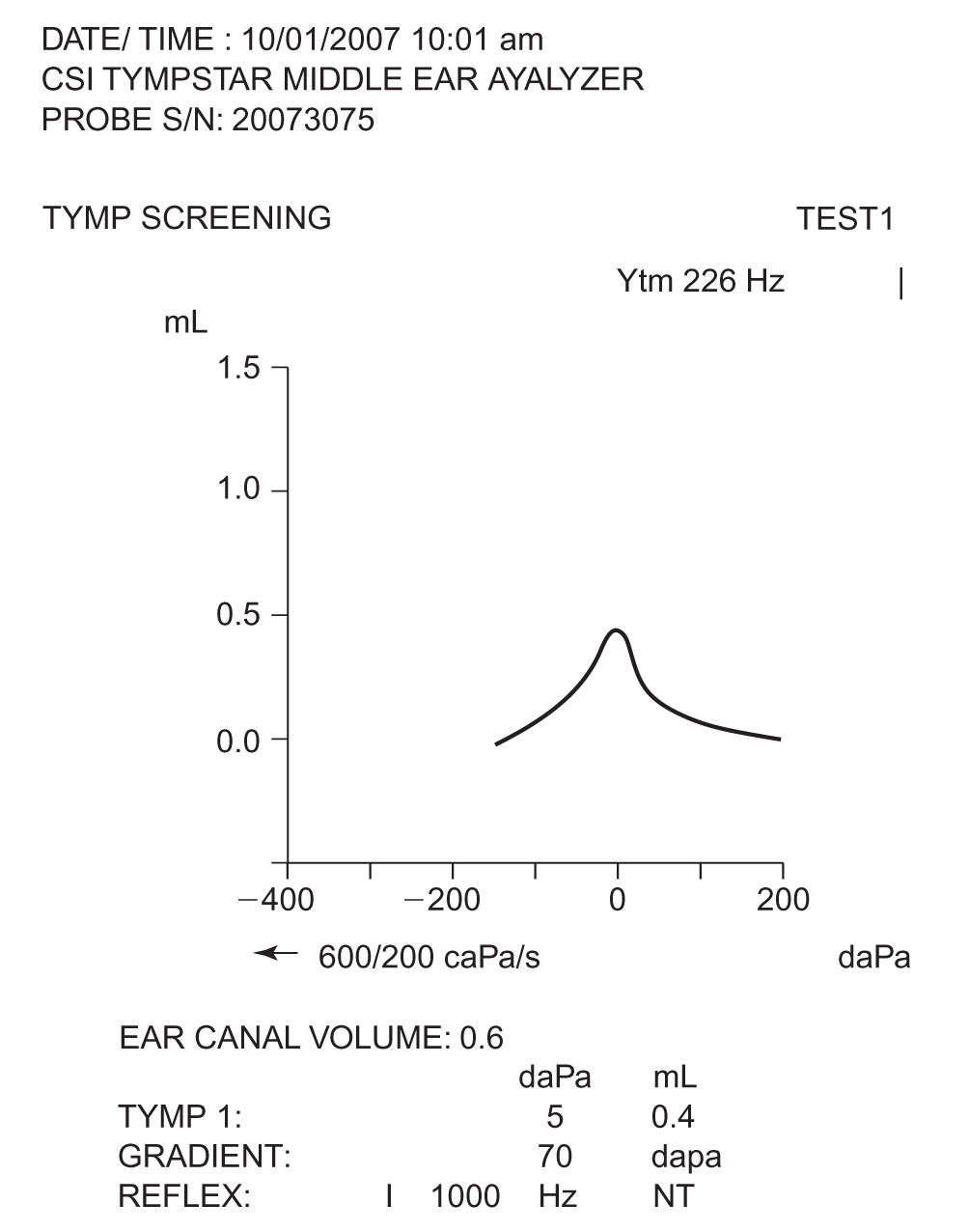
FIGURE 17.2 A sample normal (Type A) tympanogram. The compliance of the middle ear system is measured as a function of the presented pressure to the external ear.
B. Auditory brainstem response (ABR). This electrophysiologic response is generated by activation of the neurons within the eighth cranial nerve and lower auditory brainstem. Rapid, short-duration acoustic signals, such as a click stimulus, can elicit this response. Because these responses are relatively small in relation to the noise (both internal and external), signal averaging techniques are used to record the electrical response of the auditory system.
1. An example of an ABR is presented in Figure 17.3. The response is judged by the presence of positive waves (I, II, III, IV, and V) occurring within a specific latency range (i.e., the time after stimulus onset that a response occurs). The latency, amplitude, and morphologic features of the responses depend on the patient’s age, the stimulus characteristics, and the recording parameters. Persons with normal peripheral ear and lower auditory brainstem system integrity have a response to clicks at intensities as low as 5 dB normal HL (nHL). When using click stimuli, the response is sensitive to the hearing status between 2,000 and 4,000 Hz. Different methods of evoked potential testing can be used to estimate hearing sensitivity outside this frequency range, but the results typically are less robust than the responses to click stimuli.
2. The test parameters and interpretation criteria for ABR depend on the nature of the questions asked by the clinicians. Because the amplitude measures are highly variable and more susceptible to artifacts, clinicians typically use latency measures to assess integrity of the system. When screening for hearing loss, the clinician examines the waveform for the presence of distinctive peaks, particularly wave V. As the intensity of the stimulus changes so too should the latency. The obtained latencies are compared with the normative values available for the type of patient. In the differential diagnosis of retrocochlear lesions, a prolonged wave I–V interpeak latency difference becomes the most sensitive indicator of this condition. Also, other prolonged interpeak latencies and interaural latency differences can be enough information for a diagnosis.
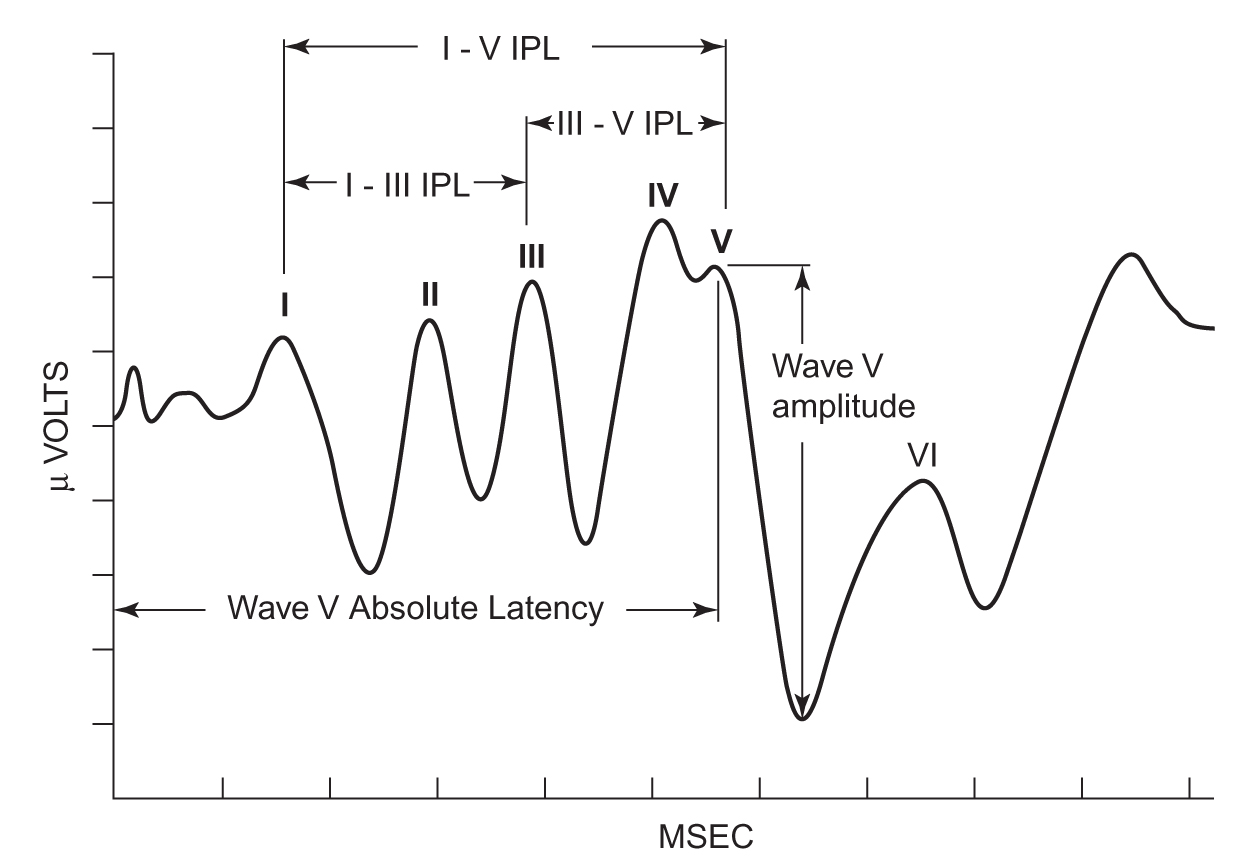
FIGURE 17.3 A sample ABR. Each wave in the response is identified using roman numerals (I, II, III, IV, and V). ABR, auditory brainstem response.
3. Although the click-evoked ABR can appear as early as the 25th week of gestation and is typically present at the 27th week of gestation, there are developmental changes in the response until approximately 2 years of age. The decrease in the absolute latency of the response is the most salient change during this maturational period. Therefore, interpretation of the ABR to identify hearing loss depends on age-appropriate norms. If wave V of the ABR is present in a test ear at 35 dB nHL, it is likely that the infant has normal-hearing sensitivity between 2,000 and 4,000 Hz.
C. Auditory steady-state responses (ASSR) are brain potentials that are evoked by steady-state stimuli as opposed to short-duration acoustic signals used to generate the ABR. The electroencephalogram (EEG) activity recorded from scalp recording electrodes contains amplitude modulations (AM) and/or frequency modulations (FM) that follow the variations in the recording stimuli. The recorded responses are suspected to arise from the auditory nerve, the cochlear nucleus, the inferior colliculus, and the primary auditory cortex because neurons at these sites are responsive to AM and FM signals.
1. The presence or absence of the ASSR is determined using statistical analyses.
2. As with the ABR, the ASSR can be used to estimate audiometric thresholds. Data have suggested that the ASSR thresholds are correlated with the ABR thresholds. Additionally, there is evidence suggesting that the ASSR can be recorded from individuals without measurable ABR. Consequently, this response has gained popularity as a tool for evaluating children who are being considered for cochlear implantation.
D. Otoacoustic emissions are sounds that are generated in the cochlea and propagate back through the middle ear and ear canal where they can be measured with a microphone. Hearing losses due to cochlear or middle ear lesions can be readily identified with otoacoustic emissions; however, these measurements do not define the severity of the hearing loss. There are two classes of otoacoustic emissions—spontaneous otoacoustic emissions and evoked otoacoustic emissions. Evoked otoacoustic emissions can be further divided according to the type of stimulus used during measurement—stimulus frequency emissions, transient evoked otoacoustic emissions (TEOAEs), and distortion product otoacoustic emissions (DPOAEs). Measurement of DPOAEs and TEOAEs is preferred for clinical purposes.
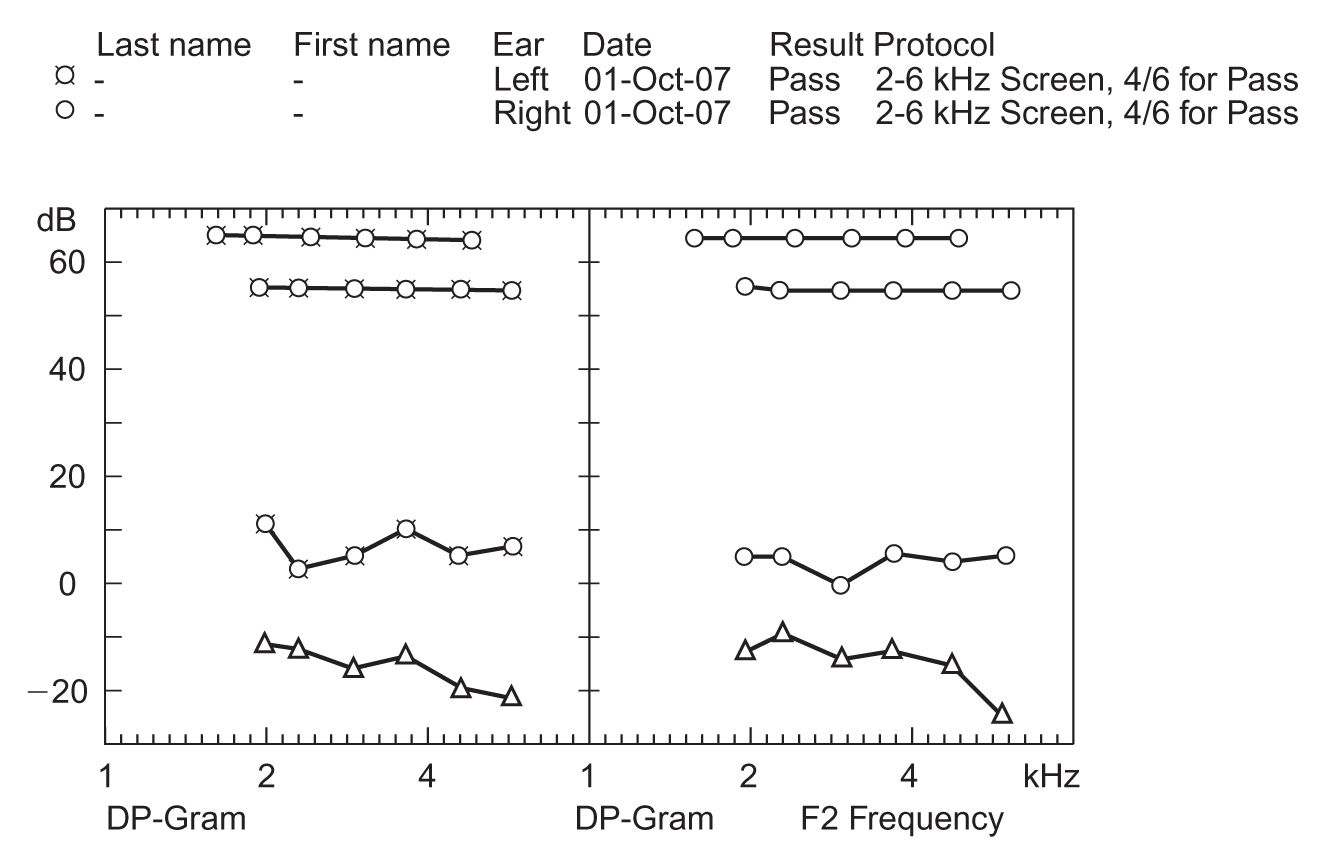
FIGURE 17.4 A sample DPOAE-gram. The left panel displays the results for the left ear (xs), and the right panel displays the results for the right ear (circles). The triangles in both panels represent the level of the noise in the ear, and the uppermost lines in each panel indicate the level of signal presentation. DPOAE, distortion product otoacoustic emission.
1. Figure 17.4 illustrates the DPOAE results from a normal-hearing adult. DPOAEs are generated from the presentation of two pure-tone frequencies to the ear, which results in a third “distortion product” response. The nonlinearity nature of the cochlea is responsible for the distortion product. In the figure, the intensity of the response is displayed as a function of the response frequency. The left panel shows the results for the left ear and the right panel shows the responses for the right ear. The triangles in both panels represent the level of the noise and the upper most lines in each panel indicate the level of signal presentation.
HEARING LOSS
It is broadly classified into two types—conductive and sensorineural—and each type has a wide variety of pathologic causes.
A. Conductive hearing loss occurs when sound cannot efficiently reach the cochlea. The blockage may be due to abnormalities of the ear canal, the tympanic membrane, or the middle ear ossicles, including the footplate of the stapes.
1. Hearing loss due to obstruction of the external auditory canal can result from impacted cerumen, foreign bodies in the canal, and swelling of the canal during infection. Cerumen impaction is the most common cause of conductive hearing loss. It is normally secreted by glands in the outer one-third of the cartilaginous portion of the ear canal. Its function is to clean and lubricate the ear canal and also to provide protection from bacteria, fungi, and insects. Other obstructions include atresia (a complete closure of the ear canal), stenosis (a narrowing of the ear canal), collapsed ear canals, and bony growths within the ear canal.
2. Conductive hearing loss may result from damage to the tympanic membrane or middle ear as a result of trauma or infection. Perforation of the tympanic membrane and ossicular discontinuity are surgically correctable.
3. Otitis media with effusion is the most common cause of conductive hearing loss among children. This condition can be associated with adenoid hypertrophy. The middle ear effusion may necessitate treatment with myringotomy (i.e., creating a small incision in the tympanic membrane) and tube placement.
4. Otosclerosis is the most common cause of conductive hearing loss among individuals in the mid-childhood to late middle-adult years. Otosclerotic bone (i.e., growth of spongy bone) progressively fixes the stapes in the oval window. This condition can be successfully managed with a stapedectomy.
B. Sensorineural hearing loss results from lesions central to the footplate of the stapes that involve the cochlea or cochlear division of the eighth cranial nerve. When the site of lesion is within the cochlea, the hearing loss is considered sensory. When the site of lesion is within the auditory neural pathway, the hearing loss is neural or retrocochlear. In sensorineural hearing losses, both air- and bone-conduction thresholds are outside the normal range of hearing sensitivity.
1. Hereditary. Sensorineural hearing loss may be hereditary; at least 100 genetic syndromes that involve hearing loss have been identified. It has been estimated that 50% of childhood sensorineural hearing loss is due to genetic factors. Genetic forms of hearing loss may be congenital or delayed in onset, unilateral or bilateral, and progressive or sudden in nature.
2. Infection. A number of viruses, including cytomegalovirus, rubella, and herpes simplex, have been implicated as etiologic agents in congenital and acquired hearing loss. Congenital syphilis and bacterial meningitis are contemporary causes of deafness despite the greatly improved treatment options available.
3. Neoplasm. In patients with unilateral progressive sensorineural hearing loss, acoustic neuroma must be suspected. Bilateral acoustic neuroma is the hallmark of neurofibromatosis type 2 and must be suspected when a patient has a positive family history (autosomal-dominant inheritance).
4. Other common causes of sensorineural hearing loss are noise exposure, metabolic and systemic changes in the auditory system, ototoxic medications, aging (presbycusis), and head trauma.
C. Mixed hearing loss exists when both conductive and sensorineural hearing losses occur in the same ear. The lesions are additive, resulting in marked air–bone gaps with the bone-conduction thresholds falling outside the normal range of hearing sensitivity.
D. Auditory neuropathy/dyssynchrony. Some patients have normal peripheral auditory systems up to and including the outer hair cells but have hearing difficulties. This condition can result from the absence of the auditory nerve, or more commonly, from dyssynchronous electrical responses that are sent to the brain from the auditory nerve. These patients present with a range of hearing sensitivities but typically have great difficulty understanding speech in degraded listening conditions. These patients typically have normal immittance results and normal otoacoustic emissions, yet they have anomalies in evoked potentials, particularly in the ABR, and in the behavioral response under earphones or in the sound field. The causes of auditory neuropathy/dyssynchrony vary among patients; however, the pathologic process most likely affects the inner hair cell or the auditory processing abilities of the auditory nerve and lower brainstem. Because of the possible dead regions in the cochlea or neural involvement, these patients do not respond typically to some of the traditional treatment protocols.
E. Central auditory processing disorder. Patients with this type of disorder have difficulty perceiving and appropriately using acoustic information because the central auditory system is incapable of adequately processing the signals transduced by the cochlea. Patients with central auditory processing disorders can be taught compensation strategies to improve their ability to comprehend speech. Many adults with sensorineural hearing losses also may have a concomitant central auditory processing disorder, which confounds the evaluation and management of the sensorineural hearing loss.
MANAGEMENT AND REFERRAL LISTS
When any hearing loss is suspected or identified, the patient should be referred for otologic examination and audiologic evaluation to determine the appropriate means of treatment. For conductive hearing loss, medical management is the primary course of treatment. Although many patients with sensorineural hearing loss require medical treatment and follow-up care, the primary course of management of this type of hearing loss is amplification, whether through hearing aids, cochlear implants, or assistive listening devices.
A. Amplification. Hearing aids differ in design, size, amount of amplification, ease of handling, volume control, and availability of special features. But they do have similar components, which include a microphone to pick up sound, amplifier circuitry to make the sound louder, a receiver to deliver the amplified sound into the ear, and batteries to power the electronic parts.
1. Hearing aid styles. The majority of hearing aids fall into one of four categories. The completely-in-the-canal hearing aid is the smallest and requires some form of automatic signal processing because it is difficult to manipulate controls, which are located deep inside the ear canal. The in-the-canal and in-the-ear aids sit outside the ear canal and allow manual manipulation of various controls on the hearing aid. The behind-the-ear hearing aid rests behind the ear and requires an earmold to direct the flow of sound into the ear. This style is often chosen for young children for safety and growth reasons. Also, the open-fit hearing aid (a behind-the-ear style) is a popular hearing aid for adults. This hearing aid is fit to the ear with a narrow tube as opposed to an earmold, which prevents the occlusion effect (i.e., the booming sensation of one’s own voice).
2. Hearing aid circuitry. Hearing aids are also differentiated according to technology or circuitry. Conventional analog hearing aids are designed with a particular frequency response based on the audiogram. The hearing aid has a series of potentiometers that the dispenser can adjust to approximate the values of amplification needed by the user. Analog programmable hearing aids contain a microchip that allows the aid to have settings programmed for different listening environments, such as quiet conversation in the home, noisy situations as in a restaurant, or large areas such as a theater. Digital programmable hearing aids have all of the advantages of analog programmable hearing aids, but the dispenser also uses digital signal processing to change the characteristics of the signal to maximize its frequency and intensity characteristics to meet the user’s needs at any given moment in time.
3. Implantable hearing aids. Implantable aids are typically provided for specific reasons as described below. However, new implantable middle ear hearing aids are being provided to conventional hearing aid recipients to help improve high-frequency hearing, to minimize acoustic feedback, and to improve sound quality. These devices are further described below.
a. Bone-anchored hearing aids. A device is implanted on the mastoid and once in place will use bone vibrations to stimulate the cochlea. These aids are provided for individuals with chronic conductive issues such as draining ears, a large mastoid bowl, otosclerosis, tympanosclerosis, or atresia.
b. Middle ear implantable hearing aids. These devices use an externally placed microphone coupled with an internal transducer that vibrates one of the ossicles. The mechanical energy is converted to electrical energy along the auditory pathway. These devices can be used for individuals with mild to severe sensorineural hearing losses.
c. Cochlear implants are amplification devices used to fit children and adults who have severe and profound hearing losses. Although the external processor is similar to that of a digital hearing aid, the internal components of the cochlear implant directly stimulate the neural cells of the eighth cranial nerve. Sounds sent to the external microphone are processed via the speech processor and then sent directly to the internal electrode array that is placed within the scala tympani of the cochlea. The electrical pulses are directed toward the spiral ganglion cells and the auditory nerve. The peripheral ear, therefore, is completely bypassed with this form of stimulation. Previously, unilateral cochlear implants were commonly provided, but more recently, patients are being provided with bilateral implants. Additionally, some individuals with unilateral implants find the use of a hearing aid in the opposite ear to be beneficial for communication purposes.
4. Assistive listening devices are specialized listening systems that may or may not interface with hearing aids and cochlear implants. These devices are designed to augment communication function by improving the signal-to-noise ratio during degraded listening activities. The type of assistive listening device is usually designated by the type of transmission properties the device uses such as FM systems, infrared systems, loop (wire inductance) systems, and hard-wired systems. These devices are particularly effective for listening in large-group situations such as in classrooms, churches, or public meetings. They are also effective in bridging the gap between many audio devices, such as televisions and radios, with the user’s hearing aids or cochlear implants.
B. Referral. The following organizations can assist the interested reader in locating patient education materials and appropriate otologic and audiologic service providers in their areas:
American Academy of Otolaryngology—Head and Neck Surgery
1650 Diagonal Road
Alexandria, VA 22314-2859
Phone: (703) 836-4444
Fax: (703) 683-3100
American Speech–Language–Hearing Association
10801 Rockville Pike
Rockville, MD 20852
Phone: (800) 638-8255
Fax: (240) 333-4705
American Academy of Audiology
11730 Plaza America Drive, Suite 300
Reston, VA 20190
Phone: (800) AAA-2336, (703) 790-8466
Fax: (703) 790-8631
Key Points
• Hearing loss affects almost 17 in 1,000 children under the age of 18 and approximately 314 in 1,000 adults over the age of 65.
• Hearing loss can result from anatomical/mechanical or neurologic issues.
• Both a medical and an audiologic examination are required to assess the extent and cause of hearing loss.
• Types of hearing loss include conductive (i.e., the outer and middle ear are affected), sensorineural (i.e., the inner ear or auditory nerve is affected), and mixed (i.e., the outer and/or middle ear and the inner ear are affected).
• Management of hearing loss can include the use of amplification, either through the use of a traditional hearing aid, or through the use of implantable hearing aids.
• Implantable hearing aids are surgically implanted in the mastoid bone, the middle ear, or the cochlea depending on the cause and severity of hearing loss.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







