Fig. 31.1
Axial T2-weighted MR image showing a large cavernous hemangioma in the lateral region of the left orbit causing optic nerve compression
The secondary tumors originate elsewhere and invade the orbit. These secondary tumors may be distant metastases or have an origin from the circumjacent structures of the orbit, such as craniofacial sinuses, facial skin, nasal cavity, intracranial contents, bone, muscles, vessels, or nerves outside the orbit, among others. The most common tumor of this group is the sphenoorbital meningioma [28].
Several different types of primary and secondary tumors may affect the orbit, such as:
Cavernous hemangioma
Meningioma (sphenoorbital meningioma, optic nerve sheath meningioma)
Fibrous dysplasia
Sarcoma (liposarcoma, rhabdomyosarcoma, fibrosarcoma, myxofibrosarcoma)
Carcinoma
Schwannoma
Neurofibroma (Fig. 31.2)
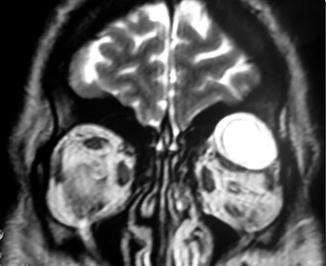
Fig. 31.2
Coronal T2-weighted MR image demonstrating a neurofibroma in the superolateral portion of the left orbit
Metastasis
Osteoma (Fig. 31.3), osteosarcoma, chondrosarcoma
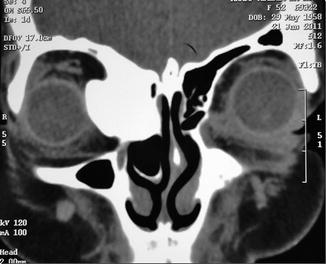
Fig. 31.3
Coronal CT scan image showing an osteoma located in the medial region of the right orbit
Pleomorphic adenoma of the lacrimal gland
Granuloma
Lymphangioma
Lymphoma
Solitary fibrous tumor
Hemangiopericytoma
Optic nerve glioma
Dermoid tumor
Esthesioneuroblastoma
Capillary hemangioma
Juvenile nasopharyngeal angiofibroma
Mucocele is a dilation of a cavity with accumulated mucous secretion. The closure of a nasal opening of a craniofacial sinus leads to mucous secretion retention. As the craniofacial sinuses are in close proximity to the orbit, the orbital cavity is frequently affected by mucoceles. The sphenoid, ethmoid, maxillary, and frontal sinuses may originate mucocele that may invade the orbit (Fig. 31.4).
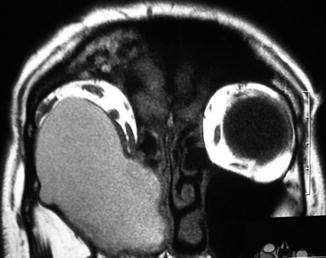

Fig. 31.4
Coronal T1-weighted MR images demonstrating a large mucocele originating from the right maxillary sinus and invading the right orbit
Orbital abscesses usually arise from circumjacent infections, such as craniofacial sinusitis, odontogenic origin, and dacryocystitis, among others. Other causes include penetrating injures, immunodeficiency, or postoperative complications.
The most frequent clinical sign of an orbital expansive lesion is proptosis, present in the majority of the patients. Other signs and symptoms are visual loss, strabismus, ocular globe deviation, diplopia, palpable masses, headache, ocular or retro-ocular pain, paresthesia, hypoesthesia or pain in the dermatome of the ophthalmic or maxillary division of the trigeminal nerve, chemosis, and pupillary abnormalities.
Patients presenting with orbital abscess may show the signs and symptoms described above, fever, and other inflammatory signs.
31.2.2 Traumatic Lesions
Traumatic lesions that may require surgery include fractures with or without bone fragments inside the orbit, blowout fractures, and penetrating injuries or foreign bodies, such as bullets of gunshot injuries. Bone fragments inside the orbit may cause ocular globe deviation, strabismus, diplopia, visual loss, and proptosis. Blowout fractures may cause diplopia, strabismus, ocular globe deviation, and enophthalmos due to orbital fat extrusion. Fractures affecting the optic canal may compress the optic nerve within the canal and cause visual loss. The degree of visual loss may vary in severity according to the degree of compression and secondary ischemia of the nerve. Unfortunately, in many of such cases, the visual loss is acute, complete, and irreversible. Retrobulbar hematomas are lesions secondary to trauma and may be visualized in the computed tomography scans. They may lead to acute visual loss due to optic nerve compromise.
31.2.3 Inflammatory Diseases
The most common inflammatory disease affecting the orbit is the orbital pseudotumor (idiopathic inflammation of the orbit). It is a non-granulomatous inflammatory process in the orbit in which a systemic or local cause cannot be found [57]. It could also be part of other diseases: Wegener granulomatosis, Erdheim-Chester disease, carotid-cavernous fistula, idiopathic meningitis, pituitary histiocytosis, systemic lupus erythematosus [38], or Tolosa-Hunt syndrome [22, 31]. The inflammatory process is heterogeneous and presents with infiltration of the retro-orbital fat, proptosis, extraocular muscle enlargement, orbital apex inflammation, and optic nerve thickening [32]. Approximately, 25 % of patients present with bilateral disease (Fig. 31.5) [34].
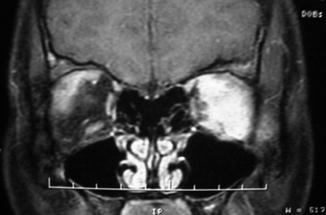

Fig. 31.5
Coronal MRI on coronal view demonstrating bilateral orbital pseudotumor, predominantly on the left side
31.2.4 Graves’ Orbitopathy
Graves’ orbitopathy is the most common extra-thyroidal manifestation of Graves’ disease and occurs in 25–50 % of patients with the disease [5, 50].
Graves’ orbitopathy may occur during or after the onset of hyperthyroidism. In other cases, the elevation of the thyroid hormones may occur months or years after the clinical signs of Graves’ orbitopathy. However, less frequently it may affect hypothyroid or euthyroid patients [21]. The disease has a self-limited active phase that usually lasts 18–24 months and abates slowly, followed by a static phase (Fig. 31.6) [27]. In the active phase, inflammation, accumulation of glycosaminoglycans, and increased fat content determine the tissue expansion within the relatively fixed space constraint of the orbital cavity [27]. The close clinical association between immunogenic hyperthyroidism and orbitopathy suggests that the antigen responsible for these diverse conditions may be shared by the thyroid gland and orbital tissues [24, 51].
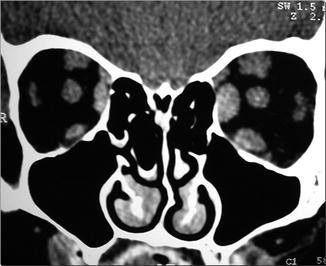

Fig. 31.6
Coronal CT scan image showing enlargement of the extraocular muscles in Graves’ disease
The diagnosis of Graves’ orbitopathy is usually made clinically. The signs and symptoms of acute phase of this disorder include proptosis, lid retraction, chemosis, corneal ulceration, diplopia, and rarely, dysthyroid optic neuropathy [37]. In the chronic fibrotic phase, the most common features are proptosis, lid retraction, and restrictive strabismus [21].
31.3 Rational Treatment Strategy
The best treatment option for orbital lesions depends on the age and clinical status of the patient, the patient signs and symptoms, the patient preference based on the surgical consent, the etiopathology of the lesion, as well its size and its location inside the orbit, and the involvement or not of extra-orbital structures.
31.3.1 Tumors
In most cases of patients with orbital tumors, the best treatment option is the surgical removal. In the cases where total tumor removal is feasible without causing neurological deficits, this should be attempted. Many tumors have a well-defined cleavage plane and can be removed with meticulous standard microsurgical technique. These well-delimited tumors include cavernous hemangiomas, capillary hemangiomas, osteomas, pleomorphic adenomas of the lacrimal gland, dermoid tumors, granulomas, schwannomas, neurofibromas, solitary fibrous tumors, cysts of microphthalmia with cyst, and others.
Although orbital capillary hemangiomas are well-delimited lesions and can usually be removed with low morbidity, their indication for surgical removal has been progressively reduced along the last years. Capillary hemangiomas usually respond to clinical treatment. Corticosteroids are used as first-line therapeutic agents for symptomatic infantile capillary hemangiomas. Intralesional steroid injections appear to cause a reduction of blood flow, with a transient reduction in volume and a suppression of the natural cyclic variation seen without treatment [56]. Other options include interferon-α and vincristine, which present problematic side effects [54]. Recently, many studies have shown the efficacy of propranolol in the treatment of this pathology [3, 46, 54]. The children are given propranolol at a dose of 2 mg/kg body weight per day for 3–10 months [54]. These lesions usually reduce significantly in size and regrowth does not occur. Based on these results, propranolol has been recently considered a first-line therapeutic agent.
Some benign tumors may have or not a well-defined limit from the orbital structures depending on the case. Sphenoid wing meningiomas with orbital invasion (sphenoorbital meningiomas) infiltrate the bone and require removal of the intracranial component of the tumor, the infiltrated bone, and the orbital part. Total resection is sometimes feasible in these cases. However, there are some findings that can preclude total removal without increasing morbidity to unacceptable levels: cavernous sinus invasion, superior orbital fissure invasion, or infiltration of the neuromuscular intra-orbital structures. In these cases, some residual tumor should be left in these structures to avoid permanent postoperative deficits. Radiosurgery or fractionated stereotactic radiotherapy may be an option to treat these residual lesions after the surgery [25, 26, 45, 60] or in the cases where tumor growing is detected in the follow-up [2, 8].
The optic nerve sheath meningioma is a different type of meningioma: it surrounds the optic nerve and is completely attached to the optic sheath. Thus, radical resection implies resection of the optic nerve. Therefore, the surgery has a limited role in the treatment of these cases if there is preoperative visual function. The surgery is reserved for cases with intracranial extension, disfiguring proptosis and patients with rapid visual deterioration. In these cases, the microsurgical approach consists in subtotal excision of the meningioma and bony decompression of the optic canal [43]. In the majority of the cases, fractionated stereotactic radiotherapy alone is the treatment of choice. Most studies report very favorable results with this modality of treatment. The reported long-term tumor control rates might approach 100 %, with greater than 80 % vision preservation or improvement after treatment [4, 7, 15, 40, 44].
Malignant tumors should be totally resected whenever possible. If radical removal is feasible with preservation of the neurovascular and muscular structures, this strategy should be carried out. If there is invasion of the intra-orbital contents, individual analyses are mandatory to define the best surgical treatment. Total resection accepting some degree of morbidity and even exenteration are possibilities to be considered.
In cases of intra-orbital lymphomas, the degree of resection usually does not improve the prognosis. In these cases, only a biopsy is sufficient to confirm the diagnosis and allow the oncological treatment. Thus, radical resection might increase the morbidity without benefits to the patient. As exception to the strategy for the treatment of orbital lymphomas described above, the primary cutaneous B-cell lymphomas should be known: the marginal zone lymphoma (MZL), the MALT (mucosa-associated lymphoid tissue) type, and follicle center lymphoma (FCL) (Santucciet al. 2012). They are mostly characterized by indolent course (very limited risk of extracutaneous spread), very good response to nonaggressive treatment, and excellent prognosis (>90 % 5-year survival overall) [48]. Although radiotherapy is the treatment of choice for these cases, radical surgical removal can lead to cure in these cases. As these subtypes of B-cell lymphomas are usually well circumscribed, surgical removal is often possible without morbidity.
31.3.2 Mucoceles
As the etiology of mucoceles is the occlusion of the communication between the craniofacial sinus and the nasal cavity, the treatment aims to drain the retained mucous secretion, to open the occluded aperture, and to keep it open.
31.3.3 Abscesses
While orbital cellulitis and subperiosteal abscesses of the orbit may be treated by antibiotic therapy alone [17], intra-orbital abscesses may require surgical drainage and postoperative intravenous antibiotic administration. Indications for surgery include pansinusitis, large abscesses with significant mass effect, concurrent intracranial involvement, poor response to initial medical treatment, and the presence of an orbital gas [62]. Sole medical treatment may work well in patients with small or medial abscesses [62].
Harris found a correlation between the age of the patient and the complexity of the disease [23]. Among patients younger than 9 years, 83 % either cleared without drainage (25 %) or had negative cultures at the time of drainage (58 %). In the culture-positive cases, single aerobes were found, and anaerobes were not isolated. Patients 9–14 years old showed a transition toward more complex infections. Patients 15 years of age or older all had positive cultures after more than 3 days of antibiotics, which were frequently effective in vitro. Thus, the age of the patient should be considered in the treatment protocol [23].
31.3.4 Traumatic Lesions
Patients with linear stable orbital fractures do not require surgery. In the cases with an exposed fracture with bone fragments within the orbit, the patients should undergo surgery for cleaning and removal of the bone pieces. In the closed fractures with bone fragments inside the orbit, surgery is indicated if the bone fragments cause symptoms and compression of the orbital structures. Fractures of the orbital floor with a blowout component should be surgically repaired. These fractures are commonly associated with diplopia and enophthalmos due to orbital fat extrusion. Thus, orbital floor reconstruction to support the orbital contents is indicated. Several materials have been used with satisfactory results: titanium, autologous bone or cartilage, flexible absorbable materials, among others [20, 53]. Prefabricated alloplastic implants together with image software and stereolithographic models have been significant advances that help to more accurately reconstruct the traumatized orbit [10].
Patients diagnosed with a traumatic retrobulbar hematoma that present with an absent relative afferent pupillary defect should promptly undergo surgery for orbital decompression. Timely operation may avoid permanent visual loss [11].
Several studies have been carried out to evaluate the therapeutic efficacy of optic nerve decompression in traumatic optic nerve orbitopathy. Although optic nerve decompression has been reported to improve the visual prognosis in some cases, its efficacy remains controversial [12, 58, 59, 63]. There is no evidence in the literature that optic nerve decompression improves effectively the prognosis in these cases. Patients with fractures in a single medial wall of the optic canal appeared to have better prognoses than patients with multiple fractures or those with a single fracture in a lateral wall [63]. The prognosis of patients without light perception that undergo optic nerve decompression for traumatic lesions is very poor [63]. Factors which predict a worse prognosis are a fracture line directly through the nerve canal and initial complete amaurosis [58]. A subgroup of patients that could benefit from this procedure is that with late onset and progressive visual loss [12]. Thus, the indication of optic nerve decompression in these cases should be individualized and further studies are necessary to establish a common sense regarding this subject.
31.3.5 Orbital Pseudotumor
The diagnosis suspicion is raised after analyzing the clinical signs and symptoms and magnetic resonance imaging findings. Systemic inflammatory diseases and Graves’ orbitopathy should be ruled out. In the cases of systemic diseases, these should be treated. In the cases of purely orbital inflammatory disorder, a therapeutic trial of corticosteroids should be used in patients with low suspicion of malignancy [6]. Patients with optic nerve compression should receive therapy with corticosteroids too. Patients with no evident diagnosis or those without response to corticosteroids trial should undergo biopsy to rule out malignancy. The patients with pseudotumor suspicion with a significant proptosis who did not respond to steroid therapy trial could be submitted to orbital decompression and biopsy at the same sitting.
The diagnosis of idiopathic orbital inflammatory syndrome (orbital pseudotumor) may be presumed, in the cases with typical imaging features and good response to corticosteroids trial, or histologically documented.
For the management of orbital inflammatory syndrome, corticosteroids were administered alone or with an immunosuppressant. In the follow up, the incidence of relapse/resistance may be higher than that of remission [1].
The therapy with corticosteroids (with or without immunosuppressant) may be maintained for several months. If the disease recurs after attempts to remove the therapy, radiotherapy may be considered.
Radiotherapy is indicated in the refractory and recurrent cases. One recent work demonstrated that 85 % with orbital pseudotumor demonstrated response to radiotherapy [41]. From these responders, 35 % had improvement in orbital symptoms without an increase in steroid dose (defined as partial response), 5 % showed complete resolution of orbital symptoms with reduction in steroid dose, and 45 % had complete resolution and achieved complete tapering of steroids. Of the group of patients who had partial response at 4 months post-radiotherapy, 35 % experienced recurrence of symptoms. Long-term complications were seen in 35 % of the patients, including four with cataract formation, one with chronic dry eye, one with enophthalmos, and one with keratopathy. Older age and complete response to radiotherapy were associated with a significantly reduced probability of symptom recurrence [41].
Fujii and coworkers reported that orbital pseudotumor might be histopathologically divided into three principal types: lymphoid, granulomatous, and sclerosing types [19]. Lymphoid and granulomatous types might be transformed into sclerosing type in the end stage. Granulomatous type indicated frequently good response to steroid and poor response to radiotherapy. Lymphoid type showed good response to radiotherapy and poor or temporary response to steroid therapy. Sclerosing type demonstrated poor response to steroid and radiation therapy [19]. Thus, biopsy might have a greater importance in the management decision of orbital pseudotumors if these features are taken in consideration.
The surgery has two main roles in the treatment of orbital inflammatory diseases: (1) to provide histological tissue for diagnosis when necessary and (2) to decompress the orbit in the refractory cases with significant proptosis.
31.3.6 Graves’ Orbitopathy
Graves’ orbitopathy should usually not be operated in the acute phase of the disease. The first step should be to control the hyperthyroidism (euthyroidism should be promptly restored). Corticosteroid or immunosuppressant agents, such as methotrexate [50], may be used. In the severe cases, weekly pulses of methylprednisolone are used and the cumulative dose should not exceed 8 g [33]. In the acute phase, surgery indication for orbit decompression might occur in two uncommon situations: (1) visual loss from orbital compression and stretching of the optic nerve. It is an infrequent but well-recognized cause of dysthyroid optic neuropathy, occurring in less than 5 % of thyroid eye disease cases [9]; (2) ocular globe subluxation in the active phase of myogenic Graves’ orbitopathy. The prevalence of this event as an indication for surgery in the myogenic variant of Graves’ orbitopathy is 0.7 % or even less [16].
In the chronic (inactive) phase, the main goal of the treatment is improvement of function and appearance. Since anti-inflammatory treatment of Graves’ orbitopathy rarely results in a complete resolution of symptoms, surgical treatment is very important for the appearance and to restore social life of these patients. Furthermore, studies have shown that visual evoked potentials have amplitude and latency significantly improved after orbital decompression [13]. Rehabilitative surgery includes orbital decompression, squint correction, lid lengthening, and blepharoplasty [14]. The orbital decompression is the surgery to be performed first, followed by the other ophthalmological surgeries in the follow up. The sequence should be as follows: surgical decompression, eye muscle surgery, and finally lid surgery [61]. Although radiotherapy might be an option as an attempt to treat the proptosis, its results are far from satisfactory when compared to surgical decompression.
31.4 Surgical Approaches to the Orbit
The choice of the approach depends on the location of the lesion in the orbit (Fig. 31.7), the size and etiopathology of the lesion, and the presence or not of involvement of extra-orbital structures.
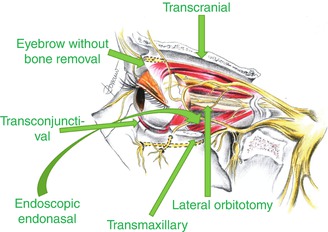

Fig. 31.7
Schematic drawing showing the entrance points of the main approaches to the orbit
31.4.1 Transcranial Approaches
The transcranial approaches used to access the orbital are (1) frontotemporal (pterional) approach, (2) frontal approach, and (3) frontolateral approach.
31.4.1.1 Frontotemporal Approach
This is the suitable via for resection of tumor lesions that invade the intracranial and intra-orbital spaces. The frontotemporal approach allows accessing the superior and lateral compartments of the orbit, the frontal and temporal cerebral lobes, the middle and anterior cranial fossae, the superior orbital fissure, and the cavernous sinus (Fig. 31.8). Wide drilling of the skull base allows approaching the inferior orbital fissure and the infratemporal fossa. Opening the superior, superolateral, and superomedial parts of the optic canal may be achieved by this via. Lesions of the orbital apex may be fully exposed through the frontotemporal access.
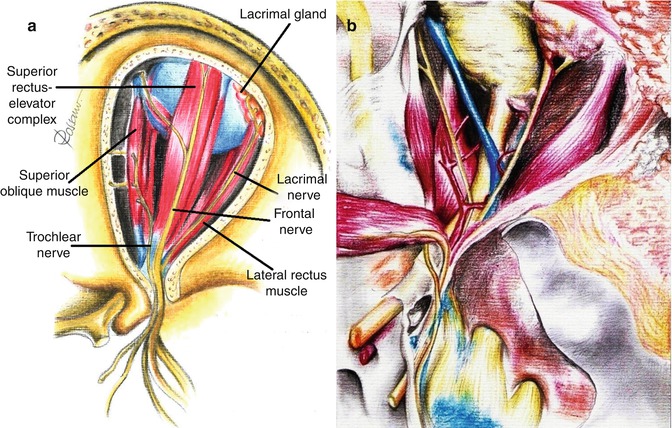

Fig. 31.8
The frontotemporal approach allows exposing the superolateral compartment of the orbit (a) and the intracranial anterior and middle cranial base, including the cavernous sinus and the superior orbital fissure (b)
31.4.1.2 Frontal Approach
Accessing the orbital roof, the superior compartment of the orbit and anterior cranial base by the subfrontal lobe via is provided by the frontal approach. If approaching the anterior cranial base is necessary, but visualization of the middle cranial fossa is not required, the frontal approach may be preferred over the frontotemporal via.
31.4.1.3 Frontolateral Approach
The frontolateral via is a small craniotomy that begins in the key hole and runs medially and ends laterally to the supraorbital nerve. Thus, opening the frontal sinus is often avoided. This via allows approaching the superior, superomedial, and superolateral parts of the optic canal. It enables opening the orbital roof and the lateral orbital wall too. Although a more angulated view is required to open the orbital roof, it can be accomplished very easily. The opening of the lateral orbital wall is performed by drilling the sphenoid wing in an in-out fashion. The exposure provided by this approach is excellent for transcranial orbital decompressions and for lesions affecting the optic canal with or without extension to the anterior skull base and central middle skull base.
Rational Use of the Transcranial Via – The personal opinion of the author is that transcranial approaches for orbital lesions are indicated when access to the intracranial structures is necessary (Fig. 31.9). The lateral and superior portion of the cavernous sinus and of the superior orbital fissure and the anterior and middle skull base can just be exposed by transcranial approaches. The orbital apex includes the transition area between the intracranial and orbital space. The superior and lateral portion of the orbital apex can only be entirely exposed by opening the optic canal and the superior orbital fissure by a transcranial approach. However, in cases where the lesion is restricted within the orbit, transcranial approaches are no longer necessary. The only exceptions are cases where orbital decompressions are necessary, such as Graves’ orbitopathy (Fig. 31.10) and orbital pseudotumor. For these cases, the frontolateral approach is an excellent option (probably the best).
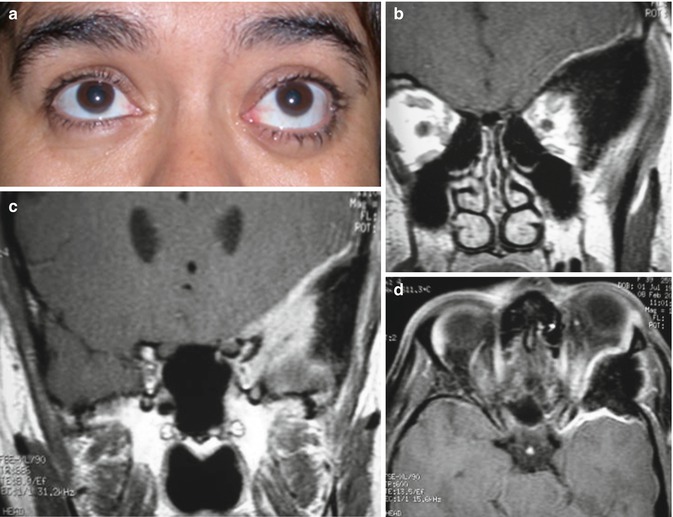
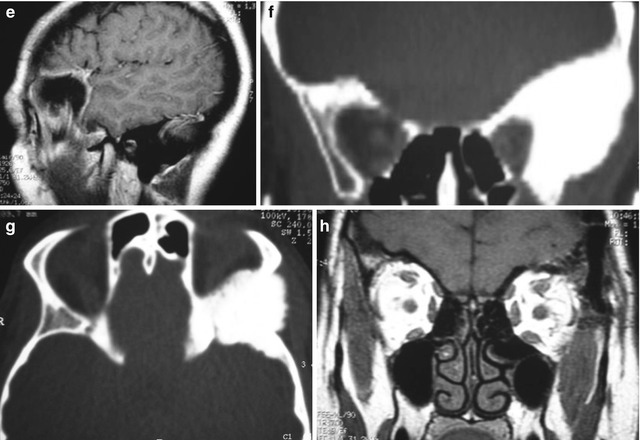
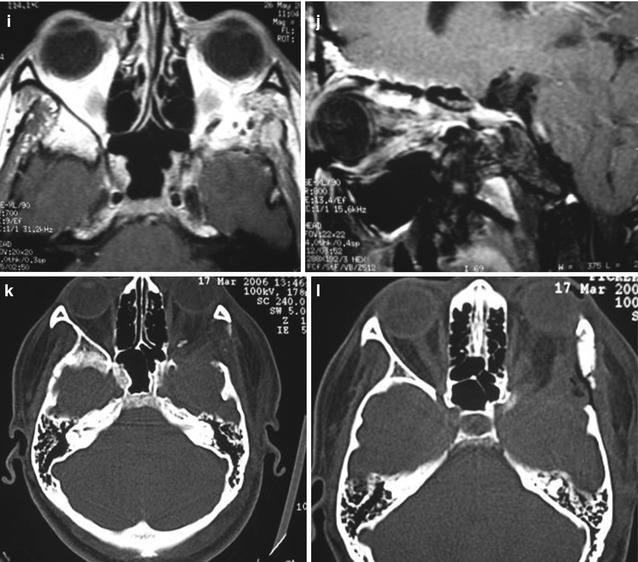
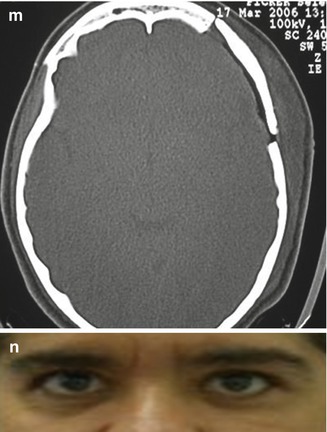
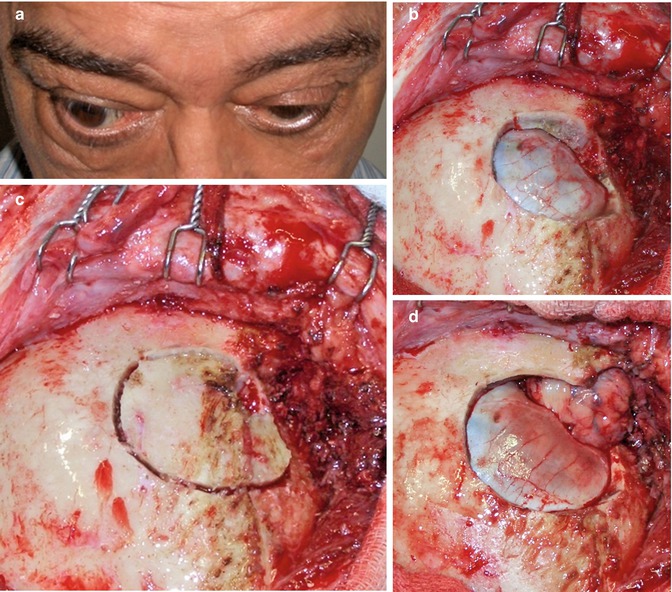
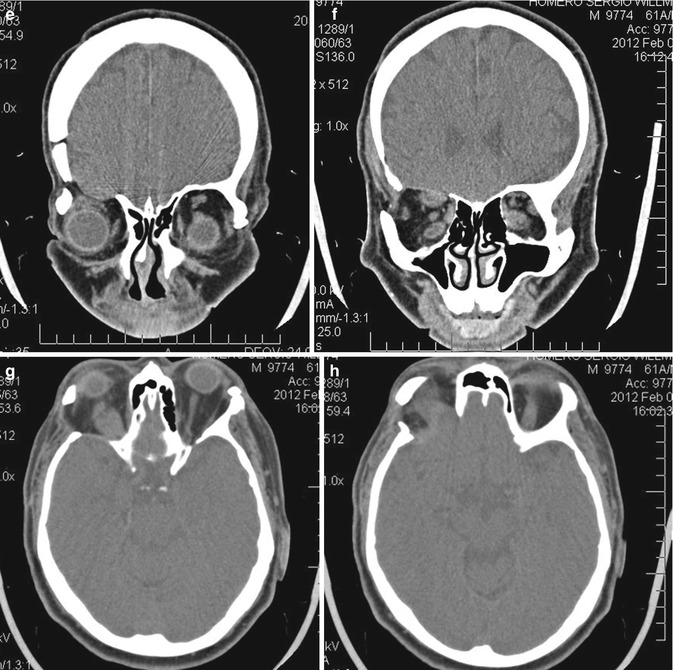
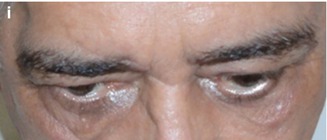




Fig. 31.9
Sphenoorbital en plaque meningioma on the left side treated by a frontotemporal approach. (a) Preoperative proptosis on the left side. (b–g) Preoperative images showing the appearance of the meningioma with orbital and bone involvement: (b, c) coronal T1-weighted MR images after gadolinium injection; (d) axial view; (e) sagittal view; (f) bone window CT scan in coronal view; (g) bone window CT scan in axial view. (h–n) Postoperative images demonstrating the complete resection of the tumor: (h) coronal T1-weighted MR images after gadolinium injection; (i) axial view; (j) sagittal view; (k, l, m) axial bone window CT scans showing complete resection of the intraosseous portion of the tumor and the cranioplasty; (n) postoperative resolution of the proptosis. The postoperative period was uneventful and there was no deficit after the surgery



Fig. 31.10
Orbital decompression performed by a frontolateral approach on the right side: (a) preoperative proptosis on the right side. (b–d) Intraoperative images: (b) frontolateral craniotomy; (c) after removing the bone flap, the orbital roof and lateral wall have to be drilled out; (d) the orbital roof and the lateral wall were removed, the superior orbital fissure was opened, and the periorbita was incised, allowing the orbital decompression of the right orbit. (e–h) Postoperative CT scans demonstrating the frontolateral approach and the superior and lateral orbital wall opening: (e, f) coronal views; (g, h) axial views; (i) postoperative improvement of the proptosis; there was no new deficit in the postoperative period
By approaching pure intra-orbital lesions by transcranial vias there are several disadvantages, such as (1) having risk of cerebrospinal fluid fistula; (2) being time-consuming compared to the other routes; (3) needing some degree of cerebral retraction; (4) having less-pleasing esthetic results due to temporal muscle atrophy and some degree of enophthalmos, since bone reconstruction of the orbital wall is usually not performed in these cases; (5) having unfavorable angle of vision; and (6) having loss of some bone anatomical references during tumor resection, thus reducing the visual and spatial orientation of the surgeon. These last two disadvantages (numbers 5 and 6) are the most important reasons why we have abandoned the transcranial approaches for pure intra-orbital lesions. The transcranial approaches make the removal of intra-orbital tumors much more difficult, due to anatomical distortion and orbital fat extrusion. The angle of vision through anterior approaches (showed below) allows a view along the axis of the nerves and muscles. Thus, nerves and muscles retractions are avoided, reducing the risks of transient and permanent extraocular muscle palsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








