Fig. 8.1
Preoperative axial, coronal, and sagittal post-contrast T1 volumetric MRI depicting 9 mm homogenously enhancing extra-axial lesion ventral to the medulla
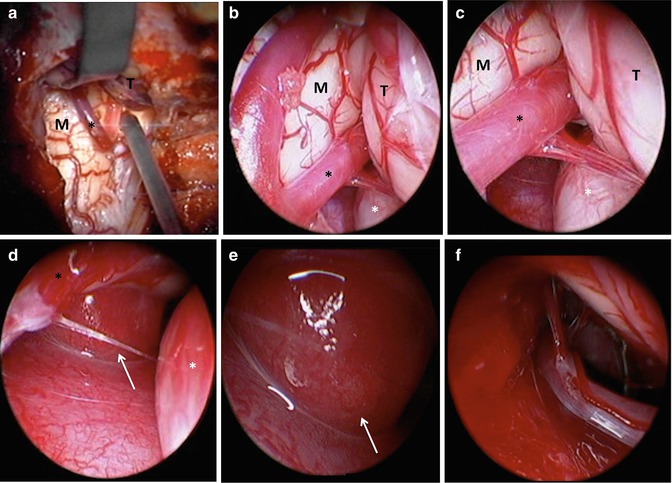
Fig. 8.2
Intraoperative photographs. (a) Microscope view depicting the medulla (M), cerebellar tonsil (T) elevated with a retractor, and posterior inferior cerebellar artery (black *) with insertion of the endoscope under direct visualization. (b–e) Depiction of progressive endoscopic views for tumor localization (white arrow) and better visualization of surrounding structures (vertebral artery = white *). (f) Endoscopic view after resection of lesion to ensure no gross tumor remnants
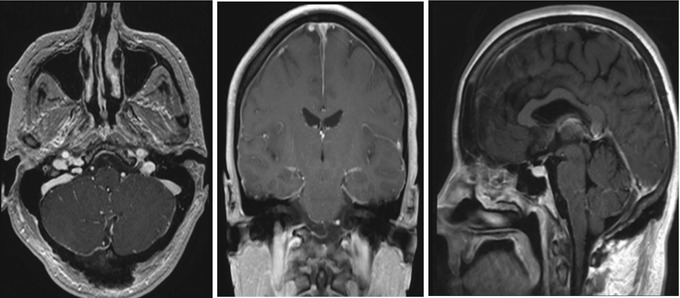
Fig. 8.3
Postoperative axial, coronal, and sagittal post-contrast T1 MRI depicting gross total resection of lesion
8.3.2 Case #2
A 75-year-old man who had previously undergone occipitocervical synovial cyst drainage presented with neck pain, dysphagia, gait imbalance, and bilateral arm numbness and weakness, greater on the left side. His physical exam revealed myelopathic gait and loss of gag reflex. MRI revealed a 1.7 × 1.4 cm anterolateral synovial cyst arising from the occiput – C1 joint on the left side with compression of the caudal medulla and upper cervical spinal cord (Fig. 8.4). Patient underwent a left C1 hemilaminectomy and resection of cyst. Significant scarring made resection difficult, and the endoscope along with the micro-Doppler was used to ensure the vertebral artery was not in danger when resecting cyst walls (Fig. 8.5). Postoperative course was unremarkable and patient was discharged after 3 days. At follow-up, patient had improvement in neck pain and motor strength. His gait imbalance was nearly resolved and was back to his usual activities.
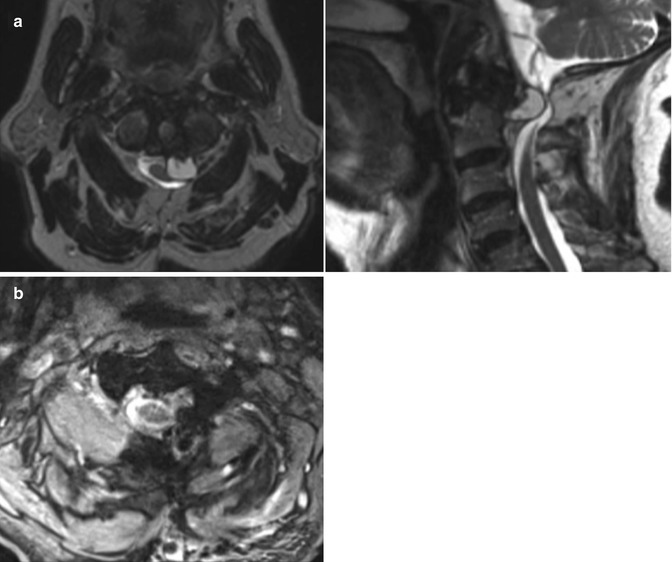
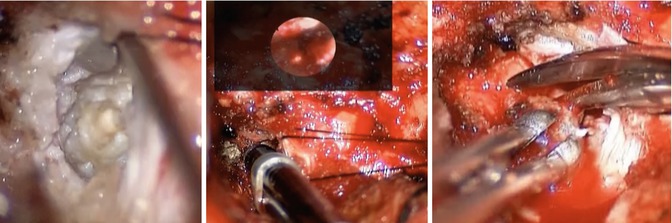

Fig. 8.4
(a) Preoperative axial and sagittal post-contrast T1 MRI demonstrating left sided anterolateral synovial cyst with moderate neural compression. (b) Postoperative view after complete resection

Fig. 8.5
Intraoperative views. Left view demonstrates white synovial cyst. Significant adhesions made resection difficult, and endoscope was used (middle view) to confirm that the vertebral artery was not present adjacent to the cyst wall during resection (right view)
8.4 Discussion
8.4.1 Classification
Utilization of endoscopic-assisted techniques in neurosurgery can be classified into three categories based on the stage of the surgery. In the initial stage, the endoscope can be used as a “look ahead” or “look around the corner” to assess key anatomical features or relationships between neurovascular and pathological structures. This is seen in the initial survey of anatomical features in pathologies such as aneurysms, vestibular schwannoma, CPA tumors, and microvascular compression syndromes. Here, one can take advantage of the endoscope’s ability to provide better illumination, higher magnification, and angled viewing. In the intermediate stage, the endoscope may be utilized as the primary form of visualization while dissecting. In some cases, the endoscope is used only for selected portions of the intermediate stage, by which the endoscope can expand the surgical working area of a given approach. For example, angled endoscopes can be used to dissect the lateral IAC in retrosigmoid [33, 34], the acom complex in supraorbital [15], or the clivus in transoral approaches [48–50]. Alternatively, the endoscope may be the only mode of visualization such as in keyhole approaches for intraventricular tumors [54–56], fully endoscopic minimal-access retrosigmoid craniotomy for microvascular decompression or vestibular schwannoma resection [40, 42, 57], or freehand posterior fossa endoscopic dissection [58]. In the final stage, the endoscope can be used as a visual tool prior to closure to assess treatment of the pathological disorder: aneurysm clip position, adequacy of microvascular decompression, or inspection for tumor remnants.
8.4.2 Visual Integration
One of the keys to maximizing efficiency and effectiveness in endoscopic-assisted microsurgery is integrating visual information from the microscope and the endoscope such that the transition is seamless and does not disrupt the flow of the operation. The ideal technology is easy to set up, slim, maximizes image quality, and allows simultaneous visualization of both endoscopic and microscopic information with minimal effort from the surgeon. Existing options include separate endoscope stand-alone monitors, mounted endoscopic displays on the wall or the microscope [59], and head-mounted liquid crystal display screen [4]. Various picture-in-picture systems exist that display two-dimensional image feeds onto a single screen that is either stand-alone or head-mounted [6]. Newer systems inject endoscopic information into the microscope oculars with the benefit of maintaining stereoscopic vision in the microscope and maintaining the flow of the operation [60, 61].
8.4.3 Instrumentation
Various endoscopes have been developed over the last 50 years. Rigid rod lens endoscopes are available in various lengths, diameters, and viewing angles. Standard viewing angles include 0°, 30°, and 45°. Increasing angles can result in disorientation and require careful manipulation under microscopic visualization since the insertion trajectory is not directly seen [7]. The ideal endoscope is thin, lightweight, sturdy, non-heat generating, and self-irrigating and can be easily integrated with a holder such that the surgeon can seamlessly transition between static and dynamic movement. Endoscopes now utilize high-definition technology, and some three-dimensional endoscope technology exists as well [62, 63]. Flexible endoscopes less than 2 mm in diameter and additional integrated fiberscope viewing dissector options exist as well [13]. Trade-offs must be made between function and optics. Smaller diameter, flexible fiberscopes rather than rigid rod lens, and some built-in irrigation systems can all decrease optical quality.
Safe endoscopic-controlled surgery requires bimanual control for fine dissection. This requires either a second surgeon if using dynamic freehand endoscope movement [58] or an endoscope holder. Various holders exist including holders secured to retractor arms and pneumatically controlled arms [55, 64].
Lastly, keyhole endoscopic-controlled approaches necessitate modified instruments that can work in smaller working channels without obstructing vision or competing with the endoscope for space. Various tubular shaft instruments exist that take up less space when opening and closing [55].
8.4.4 Future Directions
The main forthcoming advancements will be in the development of better technology to work in the current scope of indications and techniques. We will come closer to more ideal endoscopes, seamless visual integration, and more appropriate instruments. Current technology in visualization exceeds the development of endoscopic working instruments such that sometimes we have great visualization without an equivalent ability to work safely. A wider array of instruments that can function in narrow channels will likely be developed that are tailored to the needs for each indication and/or technique. If this is achieved, outcomes will improve, endoscopic-assisted indications will expand, and neurosurgeons will continue to develop more tailored individualized keyhole approaches that are equally effective and minimally traumatic.
8.5 Conclusion
The goal in neurosurgical advancement is to develop technology and techniques that allow maximal effectiveness in addressing pathology while simultaneously minimizing trauma to surrounding normal neurovascular structures. Just as the microscope provided a breakthrough in advancing these aims more than 50 years ago, so has the revival of the endoscope in the last two decades. Endoscopic-assisted techniques have become well established in the treatment of aneurysms, tumors of the cerebellopontine angle, microvascular compression syndromes, pathologies at the craniovertebral junction, and other indications. In the initial and final stages of surgery, the endoscope can provide unsurpassed visual information regarding key anatomical and pathological features and the ability for closer final inspection. In the intermediate stage, manipulation under endoscopic visualization can expand surgical working area. Technological advancements in endoscopes, holders, visual integration systems, and keyhole instruments will further the safety and effectiveness of endoscopic-assisted techniques.
References
1.
Cohen A (1996) Endoscopic neurosurgery. In: Wilkins R, Rengachary S (eds) Neurosurgery. McGraw Hill, New York, pp 539–546
2.
Gieger M, Cohen A (1995) The history of neuroendoscopy. In: Cohen A, Haines S (eds) Minimally invasive techniques in neurosurgery. Williams & Wilkins, Baltimore, pp 1–5
3.
Dandy W (1922) An operative procedure for hydrocephalus. Johns Hopkins Hosp Bull 33:189–190
4.
5.
Hopf NJ, Perneczky A (1998) Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 43(6):1330–1336; discussion 1336–1337PubMed
6.
7.
8.
9.
Kato Y, Sano H, Nagahisa S, Iwata S, Yoshida K, Yamamoto K et al (2000) Endoscope-assisted microsurgery for cerebral aneurysms. Minim Invasive Neurosurg J 43(2):91–97CrossRef
10.
Perneczky A, Boecher-Schwarz HG (1998) Endoscope-assisted microsurgery for cerebral aneurysms. Neurol Med Chir (Tokyo) 38(Suppl):33–34CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








