Artificial Disk Surgery of the Cervical Spine: Complications of Cervical Disk Replacement Surgery
Jan Goffin
The success and long-term stability of disk prostheses theoretically depend on many factors, including the prosthetic design, the materials, and the technique of insertion. The procedure should provide an immediate postoperative stable interface with the host vertebrae and, ideally, subsequent biologic ingrowth of bone to ensure long-term stability. The device should have significant strength and durability to meet the demands of the functional spinal unit in which it must function. It should be biomechanically and biochemically compatible, and it should not be associated with excessive displacement or subsidence. Experience with prosthetic large joint replacements, most commonly employing metal-on-polyethylene or metal-on-metal articulating surfaces, has shown significant problems resulting from wear debris-induced osteolysis in some cases. Since a similar problem might be encountered in the spine, minimal or insignificant wear debris should be produced by the prosthetic disk.
To provide optimal function and to avoid postoperative complications, the design objectives of a cervical artificial disk should be based on a number of principles and methods that have been validated for large joint reconstructive devices, including the following:
The device should be semiconstrained over the normal range of motion and thus be able to function synergistically with the remaining anatomic structures, such as the annulus fibrosus, ligaments, facets, and muscles.
The device should be inserted using precise bone preparation techniques, combined with porous bone ingrowth fixation. This should provide mechanical stability at the interface.
The device should provide an adequate range of motion in all relevant degrees of freedom so that normal functional motion is preserved.
The device should be able to withstand the loads and stresses encountered in the activities of daily living.
The device should be expected to have a long-term useful life in a biologic environment. Similarly, tissue ingrowth should not occur.
The device should provide elasticity and load-damping properties.
The device should be part of a system that includes instrumentation and a surgical technique that ensures accurate placement of the prosthesis with minimal resection of supporting bone and soft tissues. The surgical procedure should be straightforward and easy to perform.
The device should use materials with proven success and biocompatibility.
The device should permit conversion to fusion.
The first cervical disk was inserted in the early 1960s by Femstrom from Uddevalla, Sweden. The Bristol prosthesis has been used since the early 1990s. A greater and more widespread use, however, began with the introduction of the BRYAN total cervical disk prosthesis in 2000. Other cervical artificial disks in use today (such as the ProDisc-C, porous coated motion [PCM], PRESTIGE, etc.) became available during the subsequent years. Consequently, the longest follow-up for contemporary devices is with the BRYAN prosthesis. However, this is a mere 8 years and evaluation of long-term follow-up data, including complications, has not as of yet been reported.
In 1998, Cummins et al. (1) published their results with a series of 20 patients who were operated on between 1991 and 1996. The duration of follow-up ranged from 3 to 65 months. Seventeen patients were myelopathic and two were radiculopathic. At the end of the follow-up period, motion was demonstrated on flexion-extension radiographs in 16 patients (80%), with an average range of motion of 5 degrees. No patient required additional motion segment surgery. Radiologic examination did not demonstrate fusion at the level of the procedure in any patient, and the interspace height was preserved in all cases. Adjacent segment disk degeneration was not observed. Subsidence into the vertebral bodies did not occur, and no wear debris effects were appreciated. Osseous incorporation of the prosthesis, however, was not
demonstrated. Further technical improvements of this Cummins or Bristol prosthesis led to the development of the PRESTIGE artificial disk that is used nowadays. One of the changes was the decrease in height of both wings covering the anterior surface of the adjacent vertebral bodies.
demonstrated. Further technical improvements of this Cummins or Bristol prosthesis led to the development of the PRESTIGE artificial disk that is used nowadays. One of the changes was the decrease in height of both wings covering the anterior surface of the adjacent vertebral bodies.
Disk replacement surgery is a modern alternative for anterior cervical interbody fusions (with or without instrumentation). The philosophy for using an artificial disk in the cervical spine is different from the lumbar spine: whereas in the lumbar spine the primary goal is to treat back pain, similarly to what some want to do with lumbar fusions, the first goal of inserting a cervical artificial disk is to prevent accelerated adjacent-level degeneration, as is seen after anterior cervical interbody fusions (2, 3 and 4).
Complications with cervical disk replacements can be divided into the five categories:
Intraoperative and immediate postoperative complications
Early postoperative complications
Intermediate follow-up complications
Long-term follow-up complications
The author must confess to a lack of personal experience with devices other than the BRYAN disk replacement. However, other authors have published cases and series of complications with other devices, which serve to enrich this discussion. Some modes of failure still remain a matter of theory, but they should not be dismissed outright.
INTRAOPERATIVE AND IMMEDIATE POSTOPERATIVE COMPLICATIONS
The intraoperative risks of cervical artificial disk surgery are not substantially different from those that are known from anterior discectomy and fusion procedures, with or without instrumentation.
In the European prospective multicenter trial, with which the use of the BRYAN disk was clinically launched in January 2000 (5,6), 103 single-level and 43 bi-level patients were enrolled. No major complications were encountered. In the single-level study, one patient required reoperation after a few hours because of prevertebral hematoma causing respiratory problems. In the bi-level study, one patient experienced a dural tear and cerebrospinal fluid leak during the decompression; one patient underwent reoperation for the evacuation of an epidural hematoma; one for a prevertebral hematoma; and one for the repair of a pharyngeal/esophageal wound incurred during intubation.
The author was informally informed about a case outside the European clinical trial in which the vertebral artery was damaged during the act of transverse milling of the end plates. As is the case with most vertebral artery injuries, this was most likely due to a lack of accurate orientation to the midline of the spine. An abnormal tortuous course of the vertebral artery with a loop that extended more medially than normal might have been another explanation. Precise marking of the midline and preoperative assessment of the position of the vertebral artery on a computed tomography (CT) or magnetic resonance imaging (MRI) scan may prevent this kind of complication.
Heller et al. (7) did not encounter any major complications with the BRYAN disk in a prospective, randomized, and controlled FDA-supervised multicenter clinical trial comparing BRYAN total disk replacement with anterior cervical discectomy and fusion for the treatment of single-level symptomatic cervical disk disease: 241 patients received the investigational device and 221 patients underwent a fusion.
Similarly, Murray et al. (8) did not encounter any major complications with the ProDisc-C in a prospective, randomized, and controlled FDA-supervised investigational device exemption clinical trial of ProDisc-C cervical total disk replacement versus anterior cervical discectomy and fusion for the treatment of single-level symptomatic cervical disk disease. Two hundred nine patients were enrolled in the study, of which one hundred three underwent disk replacement surgery and one hundred six underwent a fusion. One investigational patient sustained a dural tear.
However, there have been case reports of adjacent vertebral body sagittal midline fractures due to the presence of the midline keels at the cranial and caudal sides of the ProDisc-C prosthesis. In the case of a bi-level or multilevel case, the intermediate vertebral body might be more at risk for this type of complication (9). A posterior avulsion fracture at the adjacent vertebral body, which was incurred during cervical disk replacement surgery with the ProDisc-C, has also been described (10).
With regard to the PRESTIGE cervical disk, Porchet and Metcalf (11) published the preliminary results of a European prospective, randomized, clinical trial in 2004. Twenty-seven patients were included in the PRESTIGE II disk group, and twenty-eight were included in the control group (fusion with autograft). Nineteen adverse events occurred in the control group and seventeen in the investigational group, which means no statistical difference. There were no major complications. One investigational case had a transient recurrent palsy, and one patient reported moderate dysphagia for a duration of 3 months.
Similarly, a 2-year prospective, randomized investigational device exemption (IDE) study of the PRESTIGE ST cervical disk prosthesis did not mention any severe intraoperative adverse events (12) nor did a study with the PCM (Cervitech) disk (13).
Outside the context of the European PRESTIGE cervical disk trial, the author was informed of a European case in which a central cord lesion was detected after the operation. Postoperative imaging did not reveal any source of spinal cord compression. Some MRI evidence of myelomalacia, however, was observed at the operated level. Some have speculated that such a complication might be possibly due to the so-called water-hammer effect. Gentle insertion techniques for a prosthesis might prevent this severe complication.
From a theoretical viewpoint, spinal cord and nerve root damage, as well as wrong-level surgery, etc., may thus be possible, similarly to the situation with interbody fusions.
The risk for postoperative dysphagia seems to be lower with cervical disk arthroplasty surgery than with interbody fusions. Riley et al. (14) reported a comprehensive study on the incidence of dysphagia collected by the members of the Cervical Spine Research Society: 19.8% of single-level fusion patients complained of postoperative dysphagia,
33.3% of bi-level, and 39.1% of three- or more-level cases. This incidence is higher than for BRYAN, PCM, and ProDisc-C disk replacements and may be due to the fact that these disk replacements are contained within the disk space and do not project anteriorly toward the esophagus and that they do not use screws that can back out into the soft tissues in the neck. In addition, all three do not require retraction significantly across the midline, unlike the placement of a contralateral screw to attach an anterior cervical plate (15).
33.3% of bi-level, and 39.1% of three- or more-level cases. This incidence is higher than for BRYAN, PCM, and ProDisc-C disk replacements and may be due to the fact that these disk replacements are contained within the disk space and do not project anteriorly toward the esophagus and that they do not use screws that can back out into the soft tissues in the neck. In addition, all three do not require retraction significantly across the midline, unlike the placement of a contralateral screw to attach an anterior cervical plate (15).
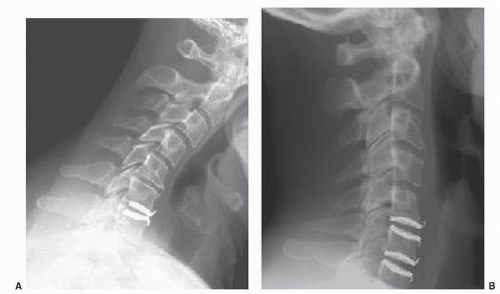
Malpositioning of the prosthesis may occur due to procedure-related or to surgeon-related problems. The classical learning curve must be taken into account (Fig. 96.1A,B). It seems probable that inaccurate placement may influence the clinical outcome or device wear. Eventually, it might disturb the overall biomechanics at the operated level and at adjacent levels, thus jeopardizing the most important goal of artificial disk surgery, namely, the prevention of accelerated adjacent-level degeneration, as observed after interbody fusion. A device might be inserted too far laterally from the midline, which could also endanger the nerve root or vertebral artery (Fig. 96.2). A device might be inserted with malrotation in the coronal plane (Fig. 96.3). Though not a desirable radiographic appearance, one would hope that the unintended shear load created by the implant position would not substantially alter the durability of the device. One could also have a mismatch in the angle of device insertion with the sagittal plane angle of the native
disk space (Fig. 96.4). Inappropriate positioning of the prosthesis with regard to the anterior and posterior borders of the disk may occur. Figure 96.5 illustrates an example of failure to insert the device deep enough into the disk space. Interestingly, this did not appear to affect the device’s function or the clinical results at 2-year follow-up.
disk space (Fig. 96.4). Inappropriate positioning of the prosthesis with regard to the anterior and posterior borders of the disk may occur. Figure 96.5 illustrates an example of failure to insert the device deep enough into the disk space. Interestingly, this did not appear to affect the device’s function or the clinical results at 2-year follow-up.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree