Posttraumatic Syringomyelia
Sean Barry
Sean D. Christie
As the prevalence of spinal cord injuries (SCIs) increases worldwide with global population growth, so too does the life expectancy of this patient cohort. Advances in medical and surgical management of these patients have led to improvements in outcomes, survival, and quality of life. This is mainly due to the focus on prevention of complications resulting from SCI, rather than any revolutionary treatment of the injured cord itself. For a patient in the third or fourth decade of life sustaining an SCI, the median survival has increased 38 years postinjury with 43% of these patients surviving for at least 40 years (1). This is in stark contrast to data reported from World War I, when 80% of patients did not survive 3 years postinjury (2). The improvement in survival has resulted in clinicians encountering novel challenges when treating patients with SCI decades following their initial trauma. An increasingly recognized delayed complication of SCI is syringomyelia and the associated tethering of the spinal cord to extramedullary soft tissues. Posttraumatic syringomyelia (PTS) was first well described in a literature review in 1898 by Wagner and Stolper, who wrote of a patient following thoracic and lumbar spinal fractures who had developed “thickening and adhesions of the meninges with each other and with the wall of the vertebral canal.” They go on to say that “we are convinced that traumatic intramedullary cavitation is the direct result of trauma. The forcible kinking of the spine will stretch the cord, and this together with slight compression at the moment of violence will lead to the formation of delicate clefts and fissures. Individual bundles of nerve fibers will be loosened and disrupted in that context. It is not inconceivable that this may happen without any extravasation of blood. In those cases the clinical picture following the lesion may be so slight as to be virtually missed. Not infrequently, however, there may be major hemorrhaging into these cavities and this will very rapidly produce a clinical picture of hematomyelia. Either way, this may be presumed to stimulate the proliferation of the glia.” These observations, made over a century ago, are extraordinarily discerning, and it is humbling how little we have learned since then (3).
Syringomyelia remains poorly understood with respect to etiology and is a notoriously challenging condition to treat, more often then not resulting in unsatisfactory outcomes. The neurologic deficits incurred from the development and progression of these cerebrospinal fluid (CSF) filled cysts are often irreversible. Furthermore, these patients often require maximal rehabilitation thus increasing the personal, social, and economic burden of this population. The goal of surgical intervention is, in general, to halt the progression of symptoms with recuperation of function and symptom alleviation often viewed as an added benefit. The intent of this chapter is to review both existing research into the pathogenesis of this condition as well as the current classification of PTS and spinal cord tethering. Guidance with respect to diagnosis as well as both conservative and surgical management of these conditions will be addressed.
TERMINOLOGY
Much confusion surrounds the various terms describing cavitations of the spinal cord. Syringomyelia, hydromyelia, syringohydromyelia, and hydrosyringomyelia have all been used to describe this condition. Cystic cavitation of the spinal cord was initially described by Estienne in 1546 in La dissection du corp human (4). The term syringomyelia originates from the Greek word surinx, meaning tube or channel, within muelos, meaning marrow. Hydromyelia is conventionally reserved to depict cystic cavitations or enlargements confined to the central canal of the spinal cord, in contrast to syringomyelia, which encompasses all extracanalicular cavitations. Histologically, hydromyelia has been distinguished from syringomyelia in that the former’s inner surface is lined with ependyma, whereas the cystic cavity in syringomyelia is generally surrounded with cells of glial origin. However, syrinx cavities can communicate with the central canal, and an area of ependymal lining has been described in the region of the syrinx in proximity to the connection. Syringohydromyelia and hydrosyringomyelia are archaic and ambiguous terms and should be considered only for historical purposes. A further descriptive term that was in vogue for a time in the literature and still used by some authors is progressive posttraumatic cystic myelopathy. This descriptive term was proposed to illustrate the progressive nature as well as the distinctive etiology of PTS. A further qualifying term is pseudosyrinx, which describes those cavities associated with intramedullary neoplasm or vascular malformation. Communicating syringomyelia are those cysts
in continuity with the fourth ventricle, whereas cervical cysts that extend into the medulla are termed syringobulbia.
in continuity with the fourth ventricle, whereas cervical cysts that extend into the medulla are termed syringobulbia.
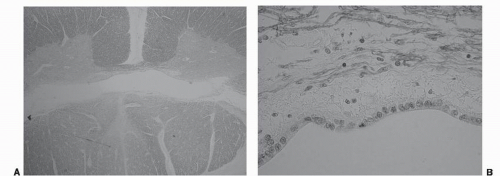 Figure 50.1. A: Low-powered H&E stain in a patient with syringomyelia. B: High-powered magnification demonstrates a single layer of ependymal cells enveloping the central cavity. |
For the sake of simplicity, all dynamic fluid-filled cavitations of the spinal cord will be referred to as syringomyelia. Those subsequent to SCI will be termed PTS. This is the terminology adhered to by most recent publications on this matter.
HISTOLOGIC CLASSIFICATION
Detailed histologic study of postmortem syringomyelia suggests important anatomical and histopathologic distinctions between the different types. Three commonly observed categories of cysts have been well described by Milhorat et al. (5) in an exhaustive and definitive postmortem study of over 100 patients. These include communicating canalicular, noncommunicating canalicular and canalicular syringomyelia. Two less commonly encountered types of syrinx, atrophic cavitations and those associated with mass lesions (pseudosyrinx) are also described.
 Figure 50.2. A: Low-powered axial H&E stain in cervical cord PTS demonstrating a paracentral white matter cavitation. B: High-powered image demonstrating the glial-lined syrinx cavity. |
Dilatations of the central canal that communicate directly with the fourth ventricle are derived predominantly from hydrocephalic patients. These cavities are lined wholly or partially by ependyma (Fig. 50.1A and B). Occurring caudal to these are tubular cavities not in communication with the fourth ventricle. These cysts are associated with disorders of CSF dynamics, most commonly at the level of the foramen magnum, and include the Chiari I malformation and basilar impression. Stenosis of the central canal, both rostral and caudal to these cysts, is characteristic. These noncommunicating cavities are more labile and have a greater propensity to dissect paracentrally into the spinal cord parenchyma than do their communicating counterparts. A third category of cyst is associated with predominantly extracanalicular parenchymal injury most often seen as a result of infarct, hemorrhage, and especially trauma. Extracanalicular syringomyelia is strongly coupled to early myelomalacia on magnetic resonance imaging (MRI). Both extracanalicular syrinxes and the parenchymal dissections of central canal syringes are lined by glial tissue (Fig. 50.2A and B). They are characterized
by the presence of central chromatolysis, neuronophagia, and wallerian degeneration (5).
by the presence of central chromatolysis, neuronophagia, and wallerian degeneration (5).
ETIOLOGY AND EPIDEMIOLOGY
Blunt trauma is the most common cause of severe SCI and hence the most commonly implicated mechanism for PTS. Indeed, trauma is the second most common cause of syringomyelia after disorders affecting the foramen magnum (6). The vast majority of these injuries are sustained from motor vehicle collisions, with a much smaller percentage originating from falls, sporting injuries, or other causes. PTS resulting from penetrating injuries such as gunshot wounds and stabbing has also been observed. Less common, although recognized, is the development of PTS following surgical resection of an intramedullary tumor, arteriovenous malformation or other mass lesion.
Nearly 81% of all SCI occur in males; however, the expression of syrinx development appears to be related to mechanism of injury, rather than gender. One series reported 88% of PTS occurring in males, which reflects the disproportionate number involved in trauma (7). SCIs occur most often in the cervical and cervicothoracic spine and the location of PTS reflects this as well. SCI principally affects young adults, although the demographics are shifting. From 1973 to 1979, the average age at injury was 28.7 years. Since 2005, the average age at injury has increased to 40.2 years (8).
Although uncommon, the development of a syrinx is a potentially devastating consequence of trauma. Approximately 11% of all syringomyelia are posttraumatic in origin. Large cohort studies have suggested that 3% to 4% of patients with SCI will at some point develop clinically apparent syringomyelia (9). A greater percentage of patients have radiographically detectable, albeit clinically imperceptible lesions. These will generally remain stable, both clinically and radiographically, over time.
PATHOPHYSIOLOGY
The pathophysiology of PTS remains inadequately understood. Most authors believe that an interrelated combination of many factors is implicated in the development and subsequent enlargement of syringomyelia after SCI. These factors include spinal instability with ongoing compression of the spinal cord, arachnoiditis leading to meningeal adhesions and cord tethering, spinal canal stenosis, and microcystic degeneration and gliosis of the injured spinal cord segment.
In the vast majority of cases, syringomyelia formation is a secondary result of an initial spinal cord insult, be it a Chiari malformation, hydrocephalus, basilar invagination, mass lesion, or trauma. There has been a significant amount of research in attempt to elucidate the etiology of syringomyelia associated with many primary neurologic disorders. It is currently widely accepted that cysts in association with Chiari malformation stem from the aberrant flow of CSF at the cervicomedullary junction resulting from tonsillar impaction at a level below the foramen magnum.
Syringomyelia associated with conditions other than Chiari malformation have no firmly established surgical interventions and pose a uniquely difficult entity. Presumably, the radiographic and anatomical similarities between cysts associated with Chiari malformation and those stemming from other primary conditions evolve from a similar pathophysiologic process, although this has not yet been definitively confirmed. As a result, the surgical philosophy employed in managing syringomyelia is similar to that for Chiari malformation: that is to attempt to restore normal preinsult CSF flow dynamics.
Experimental data regarding PTS originate from intraoperative observation, postmortem investigations, and MRI. More recently, animal models have provided some insight into the pathophysiology. Early hydrodynamic theories purported that arterial and respiratory pressure differentials postinjury resulted in distal flow of CSF down the central canal. The resulting obstruction in the proximal central canal then leads to a noncommunicating syrinx (5). This theory has been generally rejected, primarily based on subsequent histologic depiction of the dissimilarity between the central canal and syrinx cavities.
Several other theories have proposed that initial mechanical damage to spinal parenchyma from intervertebral disk, bony compression, or penetrating foreign body causes hematoma and cellular injury at the neuronal level. Gray matter cord edema ensues, which initially appears as T2 hyperintensity on MRI imaging. The injured cord parenchyma in turn evolves to form the primitive syringomyelic cavity. Vasogenic edema is the radiographic hallmark of SCI and is a reflection of the massive immune response and subsequent release of intracellular lysosomal enzymes and excitatory amino acids, including glutamate, into the local microenvironment. This contributes to delayed tissue injury after spinal cord trauma (10). There is often a combination of necrosis and liquefaction of intramedullary hemorrhage, which may then form the basis of the primitive syrinx cavity. Evidence also exists for gray matter herniation into white matter tracts, leading to necrosis and later cyst formation. This potential space evolves to become predominantly filled with CSF. The manner in which this takes place is the least understood and most contested point. Current doctrine suggests that the cyst consequently enlarges secondary to altered flow of turbulent CSF within the subarachnoid space. The most significant secondary mechanism resulting in cyst enlargement is tethering due to arachnoid inflammation, which results in spinal cord ischemia and also may impair circulation of CSF at the level of injury. Adhesion formation at the site of trauma is most detrimental during flexion and extension, which leads to upward and downward traction and distension of the dorsal spinal cord. In addition, subsequent arterial or venous obstruction may cause cord infarction, which is also thought to play a role in PTS development (11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







