Artificial Disk Surgery of the Cervical Spine: Theory and Concepts
Joseph D. Smucker
John G. Heller
Cervical arthroplasty devices have evolved significantly since the development of the original Bristol/Cummins device. While metal-on-metal implants similar to this device have advanced, the development of other bearing concepts incorporating metal alloys, polymers, and ceramics has also occurred. Currently, the term “cervical arthroplasty” is synonymous with the procedure “disk arthroplasty.” A number of these devices are in the process of early clinical use or are involved in U.S. FDA trials. The early data from these clinical trials are encouraging; however, there remains a need to demonstrate the viability of such techniques over time. In addition, the idea of cervical arthroplasty of the disk alone continues to ignore direct surgical attention to the dorsal elements at the index surgical level—leaving open the option for future modifications of the concept of cervical arthroplasty.
BACKGROUND
In the cervical spine, disks function in motion transfer and load bearing. These disks are coupled with their respective facets and dorsal ligaments/muscles and are oriented in a segmental fashion to create the biomechanical cervical spine. The cervical spine also serves as the protective passage for the spinal cord and vertebral arteries. Over time, disk degeneration may proceed from mild degenerative disk disease (DDD) to more significant cervical spondylosis. Traditionally, the surgical treatment for pathology in the cervical intervertebral disk has consisted of procedures that remove pathologic disk material and address the osseous and neurologic pathology in the region of the excised disk. The segmental defect created in the process then needs to be reconstructed by some means.
For patients with radiculopathy and myelopathy, anterior cervical discectomy and fusion (ACDF) is a timetested surgical intervention (1). It is the standard by which other cervical procedures are judged—the result of its high rate of clinical success in relieving pain and neurologic dysfunction. Plating techniques have diminished the need for postoperative immobilization or eliminated them entirely (2).
Adjacent segment degeneration (ASD) remains a concern related to the surgical treatment of cervical DDD and spondylosis with ACDF. This is defined by the radiographic appearance of degenerative change at levels above or below level(s) treated with a surgical intervention— typically a level immediately adjacent to a fused level. Rates of ASD have been reported to be as high as 92% by Goffin et al. (3) who wrote a long-term follow-up on patients after treatment with anterior cervical interbody fusion. There remains some debate as to the causation of ASD. The health of the adjacent disk may be affected not only by the influence of the fusion but also by intrinsic disk aging processes that remain independent of the fusion. It is most likely due to a combination of these factors, but the relative contribution of each has yet to be sorted out.
There is a clinical distinction between adjacent segment degeneration and adjacent segment disease (ASD). ASD is adjacent segment degeneration causing clinical symptoms
(pain and/or neurologic disorders) severe enough to lead to a patient complaint and/or require an operative intervention (4). These definitions have not been consistent in published literature, yet a number of studies have made a consistent point of distinguishing between radiographic “degeneration” and symptomatic “disease” (3,5).
(pain and/or neurologic disorders) severe enough to lead to a patient complaint and/or require an operative intervention (4). These definitions have not been consistent in published literature, yet a number of studies have made a consistent point of distinguishing between radiographic “degeneration” and symptomatic “disease” (3,5).
ASD has been documented at a rate of 2.9% of patients per annum in patients treated with a ventral cervical fusion by Hilibrand et al. (4), and 25% of patients undergoing cervical fusion will have new onset of symptoms within 10 years of that fusion. Several investigators have focused on the recurrence of neurologic symptoms and degenerative changes adjacent to fused cervical levels (3,6). It has also been demonstrated that segments adjacent to a fusion have an increased range of motion and increased intradiskal pressures (7,8).
ACDF techniques may make use of autologous iliac crest, allograft bone, and various intervertebral “cages.” Complications associated with autologous iliac crest used in ACDF are well documented (8, 9, 10 and 11). Allograft removes the risks associated with the harvest of autograft, yet it has the detriment of having a theoretical risk of disease transmission. The issues of disease transmission and contaminated graft materials have been highlighted by allograft tissue recalls by the FDA (11). Bone graft substitutes might play a role in the future practice of ACDF, but this continues to be a minority stake in the overall graft selection of modern surgeons.
Pseudarthrosis may be encountered with ACDF procedures—an effect that is increased with the number of levels fused (1,12). Use of bone morphogenetic proteins may be an alternative grafting technology to help diminish pseudarthrosis rates in patients deemed to be at high risk for nonunion (13,14). The off-label use of rhBMP-2/ACS or INFUSE (Medtronic Sofamor Danek, Memphis, TN) has been associated with an increased incidence of swelling complications and concerns for graft resorption and migration of interbody implants (15, 16, 17 and 18).
Cervical disk arthroplasty is intended to preserve motion and minimize the limitations of fusion, and may allow patients to quickly return to activities. It avoids the morbidity of bone graft harvest (19,20), concerns for pseudarthrosis, issues caused by ventral cervical plating, and cervical immobilization side effects. The procedure is designed to restore disk height and segmental motion following the removal of local pathology caused by DDD and spondylosis. The preservation of normal motion at adjacent cervical levels is a secondary intent, which may prevent later adjacent-level degeneration.
HISTORY OF DISK ARTHROPLASTY DESIGN AND DEVICE DESIGN CONCEPTS
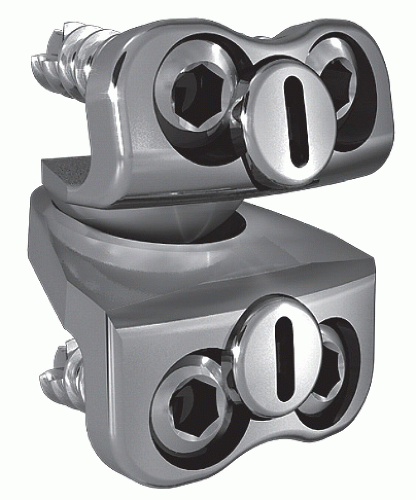
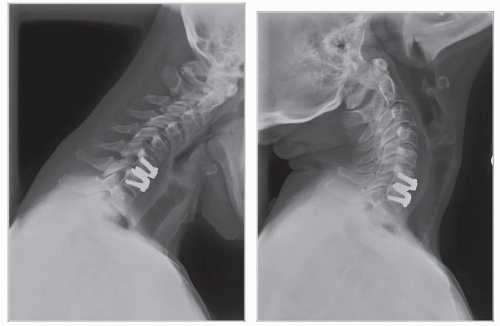
Cummins et al. (21) developed a metal-on-metal balland-socket cervical disk replacement in the late 1980s composed of 316L stainless steel. Changes to this technology from device acquisition and later development spurred a rapid transition from this device to the most recent evolution, the PRESTIGE LP (Medtronic Sofamor Danek, Memphis, TN). A predecessor of this device, the PRESTIGE ST (Medtronic Sofamor Danek, Memphis, TN), is approved for human use by the U.S. FDA (Figs. 92.1 and 92.2). Other devices in the metal-on-metal category of arthroplasty devices include the KINEFLEX-C (Spinal Motion, Mountain View, CA) and the CERVICORE disk (Stryker Spine, Allendale, NJ), which are in the process of U.S. FDA Investigation Device Exemption (IDE) trials.
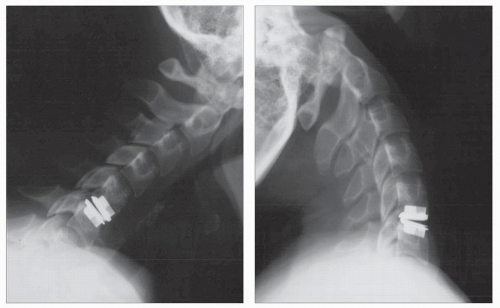
Other devices have evolved in parallel to metal-on-metal implants including the BRYAN disk (Medtronic Sofamor Danek, Memphis, TN), the porous coated motion prosthesis (PCM, NuVasive, San Diego, CA), the DISCOVER disk (DePuy Spine, Raynham, MA), and the MOBI-C (LDR, Austin, TX) (Figs. 92.3, 92.4 and 92.5). Two such devices have obtained approval for use in the US market: the ProDisc-C (Synthes Spine, Paoli, PA) (Figs. 92.6 and 92.7), and the BRYAN disk. Each of the other devices is in the process of limited human trials and/or U.S. FDA IDE submission and represents an alternative to metal-on-metal bearing surfaces, which have the potential for metal wear debris and systemic concentration of metal ions.
An overview of some concepts essential to each of these devices is presented in Table 92.1. The ideas of bearing surfaces, wear debris, and constraint are relatively young in the spine. A full understanding of the term “constraint” has not been agreed upon—constraint may arise within the device or as a result of the local anatomy (facets, posterior longitudinal ligament [PLL], interspinous ligaments, etc.). As the knowledge base in cervical total disk replacement increases, intelligent investigations and discussions will include many of these concepts and may refine our understanding of them.
The loads borne by devices in the cervical spine are far less than those in the lumbar spine. This allows engineers
to work within a more permissive design envelope for materials and other design features for cervical devices. The current devices are theoretically designed to accommodate local biomechanics and reproduce index-level motion. As intermediate- and long-term studies on individual devices become available, the design concepts of these initial devices will have the opportunity for continued examination in their in vivo environment.
to work within a more permissive design envelope for materials and other design features for cervical devices. The current devices are theoretically designed to accommodate local biomechanics and reproduce index-level motion. As intermediate- and long-term studies on individual devices become available, the design concepts of these initial devices will have the opportunity for continued examination in their in vivo environment.
CLINICAL USE CONCEPTS
INDICATIONS FOR USE
Cervical disk arthroplasty trials have included patients who were refractory to nonoperative treatment modalities with radiculopathy and/or myelopathy and with one- and two-level DDD or spondylosis (22, 23 and 24). These indications have been fairly consistent throughout the U.S. FDA approval process. At the time of this writing, three devices, the PRESTIGE-ST, the ProDisc-C, and the BRYAN disk, have achieved FDA approval for single-level use in the US market. Other devices are in various stages of the IDE and approval process (Table 92.2). ACDF should be discussed as part of the indication process for an arthroplasty procedure. The historical challenges associated with ACDF presented in this chapter may be weighed against the early nature of data with respect to cervical arthroplasty in a patient’s informed consent discussion.
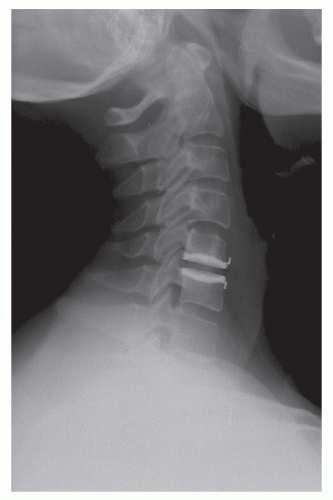 Figure 92.5. An upright lateral view of a patient who underwent successful cervical arthroplasty at C5-C6 with a BRYAN device. (© Courtesy of Medtronic Sofamor Danek, Memphis, TN; with permission.) |
In determining the indications for cervical arthroplasty of any type, it is appropriate to discuss and obtain consent for an intraoperative alternative to arthroplasty. In current practice, ACDF with plating or corpectomy and fusion with plating remain an option when it becomes clear to the operating surgeon that placement of an arthroplasty device may be compromised. The judgment to abandon the insertion of a disk prosthesis and proceed with a fusion may occur as the result of end plate defects; arthroplasty sizing/fixation issues; or other bony, vascular, or neurologic issues that would prevent the appropriate placement of the device. In some cases, this decision may simply be due to an inability to accurately visualize the operative level radiographically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree