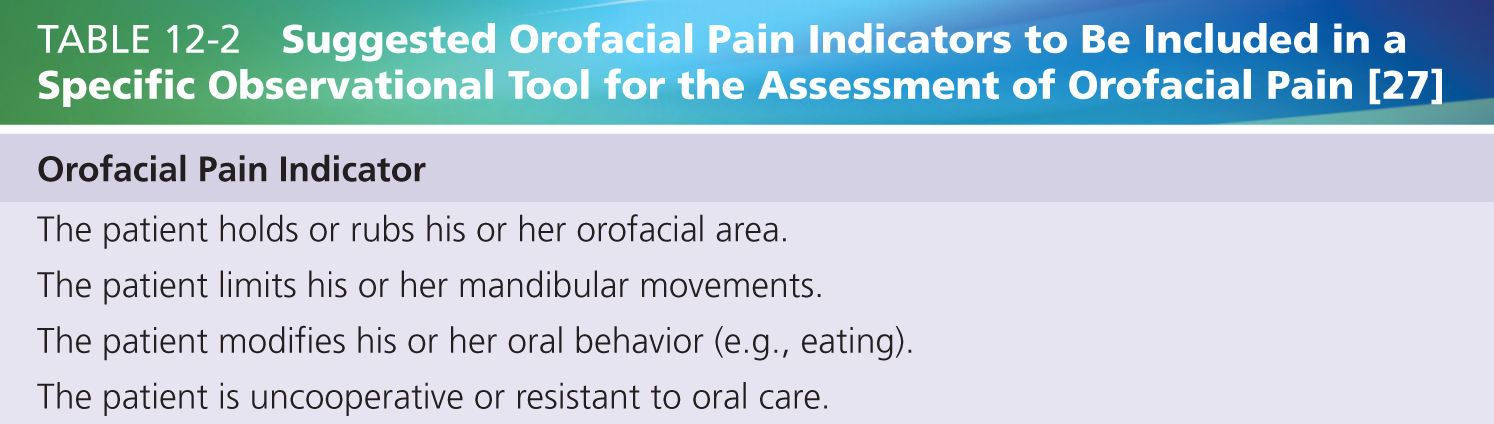
Lobbezoo et al. [27] conclude that there is a striking lack of literature on the assessment of orofacial pain in older persons with dementia, let alone that specific tools are available that can be recommended as reliable and valid instruments for such assessments. From their review, the authors did gather some suggestions as to how to compose an observational tool for the assessment of orofacial pain. The suggested orofacial pain indicators are summarized in Table 12-2. As an additional requirement, the authors indicated that the instrument should be easy to use in the elderly care clinic.
New Developments in Orofacial Pain Assessment
Although there are no papers so far that report on the development of a reliable, valid, and easy-to-use instrument for the assessment of orofacial pain in patients with dementia, some developments are worth reporting at this stage. Toxopeus et al. [43] report on the secondary analysis of the video uptakes that were used during the initial testing phase of the MOBID [17, 18], during which the teeth/mouth care item was removed for the reason stated above. Since the observers of the video uptakes during the initial testing phase of the MOBID consisted of nondental professionals only, Toxopeus et al. [43] presented the recordings to a group of experienced elderly care dentists. These observers were asked to score the video fragments for the presence of orofacial pain-/discomfort-related behaviors (viz, pain noises, facial expressions, or defense) and for the presence of dementia-related behaviors (viz, anxiety, aggression, or confusion), using the thus-composed orofacial MOBID pain scale. The scoring was done at two occasions, as to establish the reliability of the observations. Despite the vast experience of the observers in dealing with older persons with dementia, they were not able to reliably score the teeth/mouth care item in terms of pain/discomfort-related behavior or dementia-related behavior. Hence, the orofacial MOBID pain scale cannot be recommended for the assessment of orofacial pain in older persons with dementia. In conclusion, the findings of Toxopeus et al. [43] support the decision of Husebo et al. [18] to remove the teeth/mouth care item from the initial version of the MOBID.
As indicated above, the PAIC tool [5] for the assessment of pain in cognitively impaired individuals, notably those with dementia, will be the core of a comprehensive toolkit that will include additional instruments for the assessment of, among others, orofacial pain. Following the structure of the PAIC tool, a specific observational tool has been developed for the assessment of orofacial pain at rest, during oral functions like eating and drinking, and during mouth care. During these activities, observations will be made of facial activities, body movements, and vocalizations. In addition, the observer will note specific orofacial behaviors like limiting jaw movements, refusing dentures, and drooling. This tool, the Orofacial Pain Scale for Nonverbal Individuals (OPS-NVI) is currently being tested for its user-friendliness and psychometric properties by one of us (SD). Until the outcomes of that study are known, clinicians are suggested to follow the approach described by Herr et al. [11]; see Table 12-3. Importantly, since a person suffering from dementia who is being admitted to institutionalized care might already be suffering from a painful condition, establishing baseline behavior (Table 12-3) might best be done in a “guaranteed pain-free condition,” that is, while being prescribed analgesics.
OROFACIAL PAIN AND IMPAIRED CHEWING IN OLDER PERSONS WITH DEMENTIA
As indicated in the introductory paragraph, many orofacial pain conditions negatively affect the masticatory process. In turn, impaired mastication has been associated with cognitive decline. The comprehensive review of Weijenberg et al. [48] describes the evidence for this association, based on both animal studies and human work. For example, when senescence-accelerated (i.e., genetically prone to pathological aging) mice have undergone a reduced chewing intervention, like consuming a soft diet [51] or having their teeth cut [32, 45], they show impaired cognitive abilities, like reduced spatial memory function as tested in a Morris water maze (i.e., a behavioral procedure during which the mouse is placed in a large circular pool and is supposed to find a submerged platform that allows it to escape the water using visual clues). The association of this finding with older age is further underlined by the observation that the effects of reduced chewing interventions are larger in middle-aged and old mice than in young specimens [46]. Interestingly, the cognitive decline is in part reversible by fitting crowns to the cut teeth [45]. This evidence, along with the many other studies reviewed by Weijenberg et al. [48], suggests that a causal association exists between impaired chewing and cognitive decline.
Besides animal evidence for a causal relation between mastication and cognition, also human experimental studies suggest the existence of such association. In Table 2 of Weijenberg et al. [48], studies are summarized that have demonstrated, by means of brain scanning techniques like positron emission tomography and functional magnetic resonance imaging, that various brain areas (viz, prefrontal cortex, supplementary motor area, sensory–motor cortex, parietal cortex, insula, cerebellum, and thalamus) are being activated during mastication, both in young/middle-aged adults and in older persons of 60 years of age and older [31, 34].
Apart from such experimental works, Scherder et al. [36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree