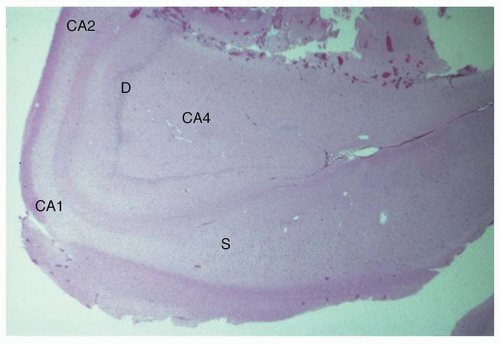
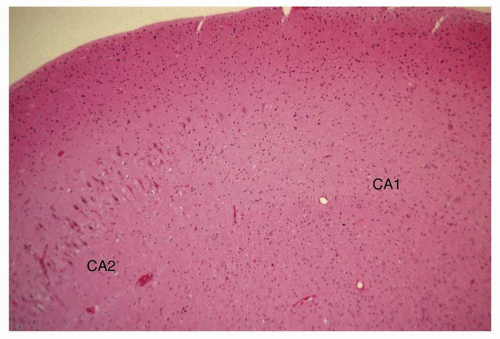
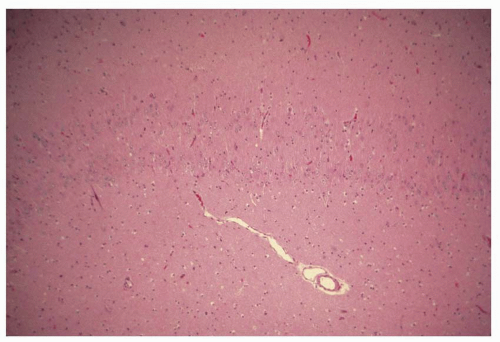
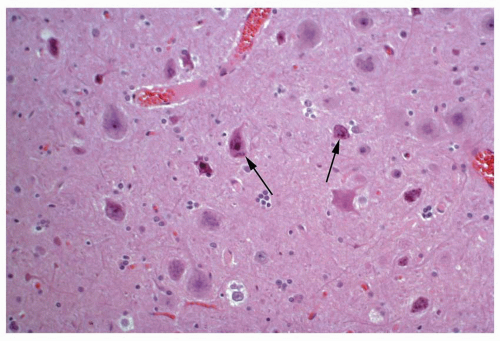
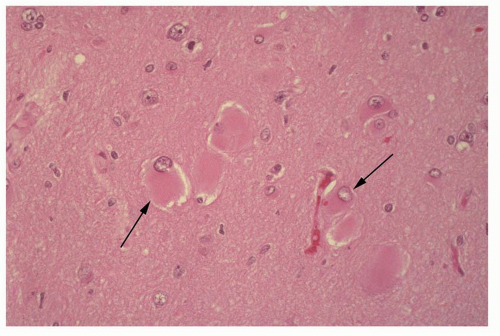
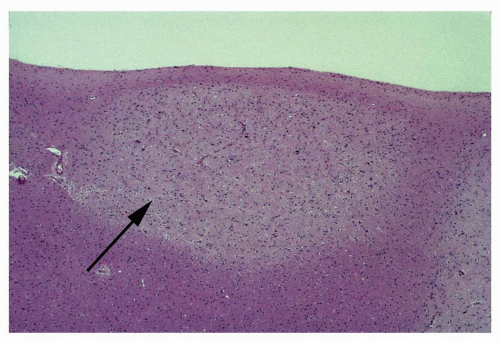
Atlas of Pathologic Substrates of Epilepsy
Richard A. Prayson
Ajay Gupta
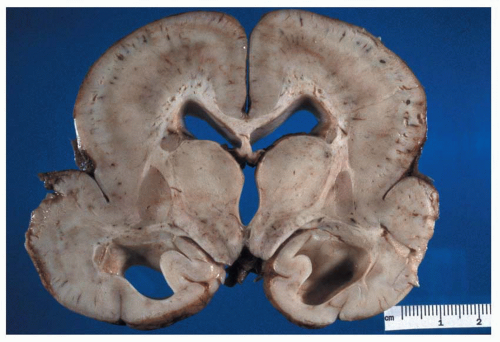
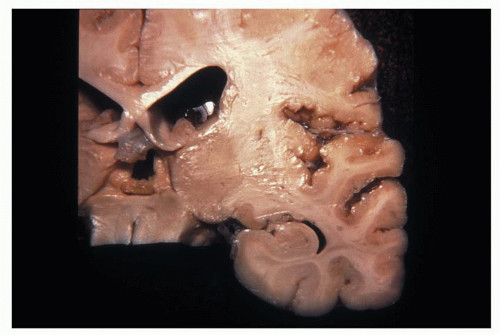
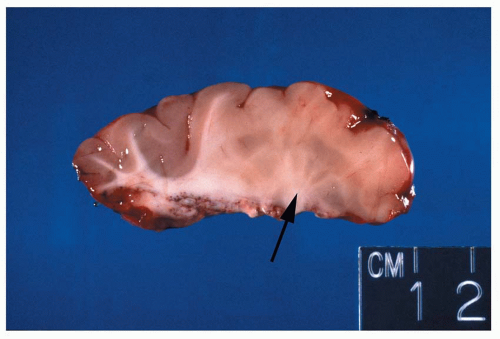
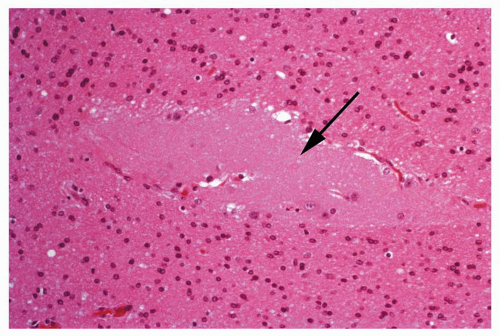 Figure 1.12 Small focus of heterotopic gray matter situated in the deep white matter of the frontal lobe region (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|