Human Nervous System Development and Malformations
Harvey B. Sarnat
Laura Flores-Sarnat
Development of the brain, both normal and abnormal, is the result of genetically determined events that occur sequentially but often with so much overlap as to be almost simultaneous. At times, abnormal development may be the residual of acquired, nongenetic lesions of the brain, such as infarcts or hemorrhages, that interfere with processes still incomplete during fetal life, but this pathogenesis of cerebral malformations is less frequent (1).
The scope of modern embryology encompasses both classical descriptive morphogenesis and molecular genetic programming of ontogenesis. The term maturation implies both growth, a measurement of physical characteristics over time, and development, the acquisition of metabolic functions, synaptic circuits, reflexes, secretory and other cellular functions, sensory awareness, motor skills, language, and intellect. For neurons, maturation includes the development of an energy production system to actively maintain a resting membrane potential, the synthesis of neurotransmitters, and the formation of membrane receptors responsive to those molecules. Membrane receptors respond to various transmitters at synapses, to a variety of trophic and adhesion molecules, and, during development, to substances that either attract or repel growing axons in their intermediate and final trajectories. Molecular genetic data are accumulating rapidly because of intense interest in this key to understanding neuroembryology, including neural induction, the influence of one immature cell or tissue on another in development (2,3).
This chapter provides an overview of development in general, with a focus on the cerebral cortex and the ontogenetic processes that may lead to abnormal architecture, or cerebral dysgenesis, which may be expressed clinically and electrographically in part as epilepsy.
NORMAL ONTOGENESIS
Maturation progresses predictably and precisely. Insults that adversely affect maturational events occur at a particular time. Some insults are brief (e.g., a single exposure to a toxin), whereas others, such as congenital infections, diabetes mellitus, and genetic or chromosomal defects, act over many weeks or throughout gestation. Even genetic disorders may be timed, however, because genetic expression is not constant throughout ontogenesis and many genes change function between early and later periods of embryonic and fetal development. Maturation of the nervous system may be summarized as a genetically programmed series of anatomic and physiologic events: gastrulation; neurulation; segmentation; neural crest separation and migration; mitotic proliferation; apoptosis; neuroblast and glioblast migration; axonal pathfinding; dendritic ramification and synaptogenesis; membrane excitability; membrane receptors; biosynthesis and secretion of neurotransmitters; myelination.
In addition to the architectural organization of the tissue in each part of the neural tube, each cell derived from neuroepithelium—neurons, glial cells, and ependymal cells—also matures and undergoes further specialization. A neuroepithelial cell, even though still in the mitotic cycle, may already have a genetic program to differentiate as a particular cellular type even before the last division has occurred, although this program may be mutable in some circumstances. A neuroblast is a postmitotic cell too immature to be considered a neuron but committed to neuronal lineage. In this context, it differs from the term blast as it is used in the hematopoietic system, for example, in which the blast cells may still be in the mitotic cycle, unlike neuroblasts. For a neuroblast to become a neuron requires an electrically polarized membrane with an energy-producing
system to maintain a resting membrane potential; synthesis of a secretory product (neurotransmitter); and membrane receptors to respond to extrinsic molecules not produced by this cell itself or to receive afferent innervation at synapses. Migratory neuroblasts to the cerebral cortex are still differentiating and may already project early axons even before migration is completed; however, because they do not yet have dendrites, synapses, polarized membranes, or secretory function, they are not yet neurons. The common term neuronal migration is technically incorrect because mature neurons do not migrate. The correct term is neuroblast migration.
system to maintain a resting membrane potential; synthesis of a secretory product (neurotransmitter); and membrane receptors to respond to extrinsic molecules not produced by this cell itself or to receive afferent innervation at synapses. Migratory neuroblasts to the cerebral cortex are still differentiating and may already project early axons even before migration is completed; however, because they do not yet have dendrites, synapses, polarized membranes, or secretory function, they are not yet neurons. The common term neuronal migration is technically incorrect because mature neurons do not migrate. The correct term is neuroblast migration.
Gastrulation and Formation of the Neural Placode
Gastrulation is the “birthday” of the nervous system, the earliest time that a neuroepithelium can be distinguished. It occurs in the human embryo when the primitive streak and Henson node form. The axes of the vertebrate body are established with bilateral symmetry—rostral and caudal (i.e., anterior and posterior) ends, dorsal and ventral surfaces, and medial and lateral aspects relative to the midline. These axes also subserve gradients of genetic expression for further development.
Neurulation
The bending of the neural placode to form a neural groove and, eventually, a neural tube depends for closure on external forces from both surrounding mesodermal tissues and surface ectoderm (4, 5, 6, 7), as well as an intrinsic component within the neural placode itself. The floor plate in the ventral midline is the first neuroepithelial region to differentiate, induced by the Sonic hedgehog (SHH) gene from the notochord (1,8). Floor plate cells assume a pyramidal or wedge shape, with a broad base ventrally and a narrow apex dorsally which faces the cavity that will become the central canal in the spinal cord; the wedge shape of each floor plate ependymal cell causes a physical bending of the two sides of the neural placode toward the midline to form a U-shaped structure and later a tube (4,8). Mechanical resistance by adhesion molecules outside the neuroepithelium also contribute to the fusion of the neural folds into a tube. The neural tube does not close at the two extremes of the placode but at some distance from each end. Beginning in the cervical region and extending both rostrally and caudally, the anterior neuropore closes at 24 days’ and the posterior neuropore at 28 days’ gestation. Located at the site of the lamina terminalis, the anterior neuropore is the origin of the telencephalon. The posterior neuropore is in the lumbosacral region; the lowest sacral and caudal sections of the future spinal cord form posterior to this site. Closure of the neural tube is not the simple bidirectional “zipperlike” mechanism once thought; several sites at each neuropore close separately (9, 10, 11, 12, 13).
The rostral end of the neural tube becomes the brain, but at no time in embryonic development is it a “cephalic ganglion.” It fulfills the criteria of a true brain from the beginning, with a bilaterally symmetric architecture, a rostral midline position, somatotopic organization of its tissue, connections with other parts of the nervous system, and a predominance of intrinsic connections by interneurons rather than external connections of primary sensory or motor neurons (14).
Cleavage of forebrain occurs at about 5 weeks gestation. This process involves a midsagittal cleft to create two telencephalic hemispheres from the single prosencephalon (cerebral vesicle) that proceded them.
Segmentation of the Neural Tube
The embryonic neural tube is genetically programmed by several families of genes (e.g., HOX, PAX, WNT, EN) to divide into compartments, or neuromeres, by physical and chemical barriers that limit the longitudinal cellular migration and facilitate the aggregation of a particular type of neuron to form nuclei or a cortex (15, 16, 17, 18). The boundaries of these neuromeres are formed by cellular processes that resemble radial glial processes and produce secretory products that repel migratory cells; mitotic activity at the boundaries of neuromeres also is less than within the neuromeres. The mesencephalic neuromere, the first distinct neuromere to appear, is sometimes regarded as a “master” that initiates the programming of other neuromere formation both rostral and caudal to the embryonic midbrain. The embryonic hindbrain divides into eight rhombomeres. Rhombomere 1 (r1), together with a posterior portion of the mesencephalic neuromere, forms the entire cerebellum except for the deep nuclei that are derived from r2. The most caudal rhombomere, r8, forms the most caudal part of the medulla oblongata and the entire spinal cord. The spinal cord only seems to be a highly segmented structure because its paired nerve roots are grouped by the segmentation of surrounding mesodermal tissues of the somites. The spinal cord is actually columnar, although there is somatotopic regionalization of neurons that is a secondary form of segmentation (19). Rhombomere 8 is also subdivided because each portion of the spinal cord is different than other regions in giving origin to either sympathetic or parasympathetic preganglionic neurons.
Rostral to the mesencephalic neuromere are six forebrain prosomeres: three diencephalic and three telencephalic (20). These six prosomeres form the various structures of the hypothalamus, thalamus, epithalamus, basal forebrain, deep telencephalic nuclei, and cerebral cortex.
The boundaries that identify neuromeres disappear after embryonic life. Neuromeres are important in the genetic programming of the central nervous system (CNS) because genes are expressed in some, but not all, of them and mediate the development of specific structures of the brain in those neuromeres. For example, in the brainstem, the
human gene ERG2 (Krox20 in rodents) is expressed only in r3 and r5, the only hindbrain segments from which no neural crest tissue migrates. HOXA1 (HOX 1.6) is expressed only in r4 to r7 and HOX2.1 only in r8. GBX2 is expressed only in r1 to r3. WNT1, EN1, and EN2 are expressed only in the mesencephalic neuromere and r1. In the telencephalon, EMX1 is expressed in the basal prosomere that forms the basal ganglia, but not in the one that forms the cerebral cortex. EMX2, by contrast, is expressed in the latter but not in the former. The selective neuromeric expression of the organizer genes is important to the normal ontogenesis of the CNS and also to its malformation. The ectopic expression of genes in neuromeres may be induced in animals by upregulation and is the basis of some dysgeneses. An example is retinoic acid (vitamin A), which upregulates certain genes; an excess produces neural tube defects and other malformations in animals and may be teratogenic in early human embryogenesis. The mutation or deletion of some genes also causes abnormal segmentation and malformation. A controversial example is the mutation of human EMX2, which results in schizencephaly (see Schizencephaly).
human gene ERG2 (Krox20 in rodents) is expressed only in r3 and r5, the only hindbrain segments from which no neural crest tissue migrates. HOXA1 (HOX 1.6) is expressed only in r4 to r7 and HOX2.1 only in r8. GBX2 is expressed only in r1 to r3. WNT1, EN1, and EN2 are expressed only in the mesencephalic neuromere and r1. In the telencephalon, EMX1 is expressed in the basal prosomere that forms the basal ganglia, but not in the one that forms the cerebral cortex. EMX2, by contrast, is expressed in the latter but not in the former. The selective neuromeric expression of the organizer genes is important to the normal ontogenesis of the CNS and also to its malformation. The ectopic expression of genes in neuromeres may be induced in animals by upregulation and is the basis of some dysgeneses. An example is retinoic acid (vitamin A), which upregulates certain genes; an excess produces neural tube defects and other malformations in animals and may be teratogenic in early human embryogenesis. The mutation or deletion of some genes also causes abnormal segmentation and malformation. A controversial example is the mutation of human EMX2, which results in schizencephaly (see Schizencephaly).
Neural Crest Separation and Migration
Almost immediately after neurulation, the tissue formed at the dorsal midline of the neural tube, shortly after closure, separates and migrates ventrally on either side of the neural tube from all rhombomeres except r3 and r5 (21, 22, 23, 24). Except for the axons themselves, this neural crest tissue forms the peripheral nerves, including the Schwann cells, dorsal root ganglia, autonomic nerves and ganglia, melanocytes, and adipose tissue. It therefore becomes the entire peripheral nervous system, including the autonomic nervous system, and differentiates into a variety of cells of both ectodermal and mesodermal germ layer origin. An important rostral neural crest migration is from the midbrain neuromere; a small prosencephalic neural crest also contributes. The prosencephalic neural crest migrates as a vertical sheet in the midline of the head. The mesencephalic neural crest tissue migrates in waves as streams of cells. These cephalic neural crest tissues form the cranial vault, facial cartilage and bone, connective tissue, blood vessels, nerve investments, and the globes of the eyes except for the retina, lens, choroid, and iris, as well as strictly neural structures such as the ciliary ganglion. The rhombencephalic neural crest migrates in streams as well, but more as blocks of cells. Many inductive genetic and growth factors are essential to neural crest development and migration, including the SHH gene, bone morphogenetic proteins (BMPs), glial cell-derived growth factor, and transforming growth factor-β (22).
Mitotic Proliferation of Neuroblasts (Neuronogenesis)
After the neural tube closes, neuroepithelial cells proliferate exponentially in the ventricular zone, with most mitotic activity occurring at the ventricular surface (Fig. 2.1). The rate of division is greatest during the early first trimester in the spinal cord and brainstem and during the late first and early second trimester in the forebrain. Within the ventricular zone of the human fetal telencephalon, approximately 33 mitotic cycles provide the total number of neurons required for the mature cerebral cortex (25). The orientation of the mitotic spindle determines the immediate fate of the daughter cells. If the cleavage plane is perpendicular to the ventricular surface, the two daughter cells become equal neuroepithelial cells preparing for further mitosis. If the cleavage is parallel to the ventricular surface, the two daughter cells are unequal (asymmetric cleavage). The daughter cell at the ventricular surface becomes another neuroepithelial cell; the other separates from its ventricular attachment and becomes a postmitotic neuroblast ready to migrate to the cortical plate. Furthermore, the products of two genes that determine cell fate, called numb and notch, are on different sides of the neuroepithelial cell. With symmetric cleavage, both daughter cells receive the same amount of each; with asymmetric cleavage, the cells receive unequal ratios of each, which also influences their subsequent development (26, 27, 28). The transcription product of numb and a related protein, numblike, maintains self-renewal properties of neural progenitor cells to generate the correct number of cells for the mature brain (29,30). Other genes influence mitotic cycling as well, and their mutation accounts for some cases of primary microcephaly and a thin cerebral mantle (see Disorders of Mitotic Proliferation and Apoptosis).
Active mitoses cease well before birth in most parts of the human nervous system, but retaining a potential for postnatal mitoses of neuroblasts are the periventricular region of the cerebral hemispheres (31) and, the best-documented site, the external granular layer of the cerebellar cortex, where mitoses persist until 1 year of age. Postnatal regeneration of these neurons, after most are destroyed by irradiation or cytotoxic drugs, is well documented in animals and probably occurs in humans as well. Primary olfactory receptor neurons also can regenerate. If they did not do so throughout life, the individual would become anosmic after a few upper respiratory infections, which transiently denude the nasal mucosa. Some undifferentiated neuroepithelial stem cells persist in the periventricular region of the mature brain, especially in the hippocampus, and might be induced to proliferate to replace neurons lost under pathologic conditions (32,33).
Apoptosis
Excessive neuroblasts are formed in every proliferative part of the embryonic, fetal, and, to some extent, the postnatal nervous system. Apoptosis, programmed cell death, restricts this overabundance until the definitive number of immature neurons is achieved (34). Apoptosis differs from necrosis, which might occur in infarcts. The many factors that arrest programmed cell death in the fetus are partly genetically
determined and partly influenced by acquired events affecting the brain or spinal cord. Cells that do not match with targets are more vulnerable to degeneration than are those that achieve synaptic contact with other cells. Endocrine hormones and neuropeptides modulate apoptosis, as do the c-FOS gene and suppressor genes such as BCL2, which inhibit the expression of apoptotic genes. Growth factors—particularly four structurally related proteins: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4)—modulate neural activity-dependent competition (35,36). NGF and BDNF are thus able to protect the neonatal brain against hypoxic/ischemic injury, an activity unique to the immature nervous system (37,38).
determined and partly influenced by acquired events affecting the brain or spinal cord. Cells that do not match with targets are more vulnerable to degeneration than are those that achieve synaptic contact with other cells. Endocrine hormones and neuropeptides modulate apoptosis, as do the c-FOS gene and suppressor genes such as BCL2, which inhibit the expression of apoptotic genes. Growth factors—particularly four structurally related proteins: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4)—modulate neural activity-dependent competition (35,36). NGF and BDNF are thus able to protect the neonatal brain against hypoxic/ischemic injury, an activity unique to the immature nervous system (37,38).
Apoptosis has two phases. The first phase involves undifferentiated neuroepithelial cells or neuroblasts with incomplete differentiation (first and second trimesters); the second phase involves fully differentiated neurons of the fetal brain and spinal cord (third trimester and postnatal period) that account for some progressive degenerative diseases such as spinal muscular atrophy. In normal development as well, mature neurons are eliminated to correct topographic targeting errors (39).
Neuroblast Migration
Neurons of the mature human brain occupy a different site from where they were generated, migrating to that site to establish synaptic connections with appropriate neighboring neurons and to send their axons in short or long trajectories to predetermined targets. The embryonic telencephalon consists of concentric layers. The subventricular zone (“germinal matrix”) contains postmitotic, premigratory neuroblasts and glioblasts that are the source of radial migration. In general, maturing nerve cells move centrifugally, toward the brain surface. The cerebellar cortex is exceptional in that external granule cells first spread over the surface of the cerebellum and then migrate deep into the folia. Neuroblast migration begins at about 6 weeks’ gestation in the human cerebrum and is not completed until at least 34 weeks of fetal life, although after midgestation most germinal matrix cells are glioblasts. Glioblasts continue to migrate until early in the postnatal period. Within the brainstem, neuroblast migration is complete by 2 months’ gestation. Cerebellar external granule cells, by contrast, continue migrating until 18 months of age (40).
Most neuroblasts of the subventricular zone or germinal matrix reach the cerebral cortical plate by traveling radially along long, slender, centrifugal processes of specialized astrocytes of the subventricular zone called radial glial cells; the neuroblasts glide on the outside of these radial glial fibers as if they were on a monorail (Fig. 2.1). Facilitating gliding of neuroblasts along a radial glial fiber are specialized proteins such as astrotactin that are secreted by the neuroblast itself (41). The L1-CAM neural cell adhesion molecule is produced in the glial cell (42); other adhesion molecules also facilitate gliding (43). Fetal ependymal cells have radiating processes that resemble those of the radial glial cell but do not extend beyond the germinal matrix and do not guide neuroblasts, although they do secrete molecules into the extracellular matrix (44,45). Some adhesion molecules of uncertain origin also reside in the extracellular matrix (46) where they serve as lubricants and adhesive factors between the membranes of the neuroblast and those of the radial glial fiber and also perhaps as nutritive and growth factors. The mechanism by which they stimulate cell movement is incompletely understood. Deficient molecules lead to defective migration. For example, L1-CAM is the defective genetic program in X-linked hydrocephalus accompanied by polymicrogyria and pachygyria (42).
The transformation of radial glial cells into astrocytes and ependymal cells begins during the first half of gestation and ends postnatally. During peak neuronal migration at midgestation, many radial glial cells remain attached to the ventricular and pial surfaces, lengthening and curving with the expansion and convolution of the cerebral wall. From 28 weeks’ gestation to 6 years of age, astrocytes of the frontal lobe shift from the periventricular to the subcortical region. The centrifugal movement of this band of normal gliosis marks the end of neuronal migration in the cerebral mantle. Ependyma does not completely cover the surface of the lateral ventricles until 22 weeks’ gestation (44).
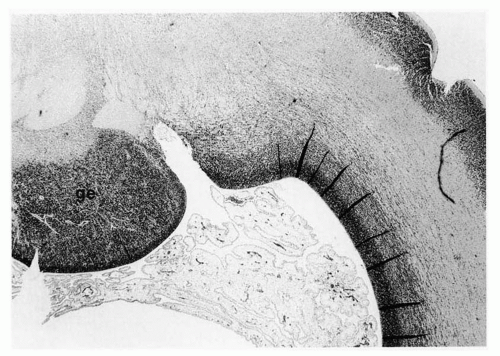
Although the number of neuroblasts is far smaller, tangential migration also occurs (47,48) perpendicular to the radial fibers. The use of axonal rather than glial guides for migratory neuroblasts explains in part why all cells in a given region of cortex are not from the same clone or vertical column (49). Most tangentially migrating neuroblasts in the cerebral cortex originate from the ganglionic eminence, a large mass of neuroepithelial cells at the base of the telencephalon that later contribute to the basal ganglia.
Tangential migrations also occur in the brainstem, olfactory bulb, and subpial region.
Tangential migrations also occur in the brainstem, olfactory bulb, and subpial region.
Growth of Axons and Dendrites
During neuroblast migration, neurons remain largely undifferentiated, and the embryonic cerebral cortex at midgestation consists of vertical columns of tightly packed cells between radial blood vessels and extensive extracellular spaces. Cytodifferentiation begins with a proliferation of mainly endoplasmic reticulum and mitochondria in the cytoplasm and clumping of condensed nuclear chromatin at the inner margin of the nuclear membrane. Rough endoplasmic reticulum swells, and ribosomes proliferate.
The outgrowth of the axon precedes the development of dendrites, and the axon forms connections before dendrites begin to differentiate. The projection of the axon toward its destination was first recognized by Ramón y Cajal, who called it cône d’accroissement (growth cone). The chemical, endocrine, or electrotactic factors that guide the growth cone to its specific terminal synapse have long been a focus of controversy; however, growth cones are now known to be guided by diffusible molecules secreted along their pathway, such as netrins and semaphorins, and others attached to the cell surface, such as ephrins (50). Diffusible molecules are secreted by the processes of fetal ependymal cells and perhaps of glial cells (51, 52, 53). Some molecules, such as BDNF, netrin, and S-100β protein, attract growing axons, whereas the glycosaminoglycan keratan sulfate (not to be confused with the protein keratin) and the protein products of the gene Slit strongly repel them, preventing aberrant decussations and other deviations (51,54). Growth cone repulsion is sometimes called “collapsing” of the growth cone. Matrix proteins, such as laminin and fibronectin, also provide a substrate for axonal guidance. Proteins within the axonal growth cone itself, such as GAP43, may serve as intrinsic signal transduction in axonal guidance (55). Cell-to-cell attractions operate as the axon approaches its final target. Axonal transport, a later stage in axonogenesis, is regulated by phosphorylation of neurofilaments, a characteristic of neurofilament maturation (56). Genetic expression of several genes influences axonal growth cone projection; one of the most important is the Wingless (WNT) family (57).
Despite the long delay between the migration of an immature nerve cell and the beginning of dendritic growth, dendritic branching eventually accounts for more than 90% of the synaptic surface of the mature neuron and is specific for each type of neuron. Spines form on the dendrites as short protrusions with expanded tips, providing sites of synaptic membrane differentiation. Neurotrophins and calcium regulation are important for synaptic plasticity (58,59).
Electrical Polarity of the Cell Membrane
Membrane excitability is an important marker of neuronal maturation, but information is incomplete about its exact timing and duration. Membrane polarity is established before synaptogenesis and before the initiation of neurotransmitter biosynthesis. Because maintenance of a resting membrane potential requires continuous energy expenditure to fuel the sodium-potassium adenosine triphosphatase (ATPase) pump, it is beyond the capability of an undifferentiated neuroblast. The formation of ion channels within the neural membrane also enables the maintenance of a constant resting membrane potential.
Synaptogenesis
Synapses form after the development of dendritic spines and the polarization of the cell membrane. The relation of synaptogenesis to neuroblast migration differs in different parts of the nervous system. In the cerebral cortex, synaptogenesis always follows neuroblast migration. In the cerebellar cortex, however, the external granule cells develop axonal processes that become the long parallel fibers of the molecular layer and make synaptic contact with Purkinje cell dendrites before migrating through the molecular and Purkinje cell layer to their mature position within the folium. Afferent nerve fibers reach the neocortex early, before lamination occurs in the cortical plate. The first synapses are axodendritic and occur both external to and beneath the cortical plate in the future layers I and VI, the latter containing the first neurons of radial migration (see Ontogeny of Cerebral Neocortex).
An excessive number of synapses form on each neuron, with subsequent elimination of redundant ones. Outside the CNS, muscle fibers also receive multiple sources of innervation, later retaining only one. Transitory synapses also form at sites on neurons where they are not found at maturity. For example, the spinal motor neurons of newborn kittens display prominent synapses on their initial axonal segment, where they are never found in adult cats. Somatic spines are an important synaptic site on the embryonic Purkinje cell, but they and their synapses disappear as the dendritic tree develops.
A structural-functional correlation may be made in the developing visual cortex. In preterm infants of 24 to 25 weeks’ gestation, the visual evoked potentials recorded at the occiput exhibit an initial long-latency negativity, but by 28 weeks’ gestation, a small, positive wave precedes this negativity. This change corresponds to dendritic arborization and the formation of dendritic spines at that time. In the development of thalamocortical connections, major synaptic rearrangements are possible only during a critically defined period and not later (60).
The electroencephalogram (EEG) of the premature infant follows a predictable and time-linked progression in maturation that has been extensively studied. Reflecting synaptogenesis more closely than any other feature of cerebral maturation, the EEG provides a convenient, bedside, clinically useful measure of neurologic (i.e., electrocerebral) maturation in the preterm infant (1,61, 62, 63).
Biosynthesis and Release of Neurotransmitters and Formation of Transmitter Receptors
The synthesis of neurotransmitters and neuromodulating chemicals is based on the secretory character of the neuron, without which synaptic transmission is impossible. Several substances serve as transmitters: acetylcholine, monoamines (dopamine, norepinephrine, epinephrine, and serotonin), neuropeptides (substance P, somatostatin, and opioid-containing peptide chains such as the enkephalins), and simple amino acids (glutamic acid, aspartic acid, γ-aminobutyric acid [GABA], and glycine). Some transmitters (glycine, GABA, and acetylcholine in the CNS) are inhibitory. Each neuronal type produces a characteristic transmitter (motor neurons produce acetylcholine, cerebellar Purkinje cells, synthesize GABA; and granule cells produce glutamic acid in the adult). Neuropeptides may coexist with other types of transmitters in some neurons. Calcium ions are a strong trigger of neurotransmitter release, regardless of the transmitter’s identity, and some synaptic vesicle proteins, such as synaptotagmin, serve as calcium sensors for this purpose (64).
In some parts of the brain, transitory fetal transmitters may appear during development and then disappear. Substance P and somatostatin are present in the fetal cerebellum at midgestation but are never found in the mature cerebellum. In the cerebral cortex of the frontal lobe, laminar distribution of cholinergic muscarinic receptors of the mature brain is the inverse of the fetal pattern. The functions of these transitory transmitter systems are unknown. Some act as trophic molecules rather than transmitters in early development. Even amino acid transmitters like GABA may have mainly a trophic function early in development. In situ hybridization and new immunocytochemical techniques demonstrate neurotransmitters in neurons of the developing brain of experimental animals, and these techniques also can be applied to human tissue (65). The ontogeny of neurotransmitter systems depends not only on the synthetic mechanisms of the specific chemical transmitters but also on the development of highly specific receptors of these chemical signals. That such receptors can modify the excitability of neuronal membranes and trigger action potentials after the recognition of specific molecules represent equally important components of the system of neurotransmission (66, 67, 68).
The development of receptors for neurotransmitters and specializations of the synaptic membrane are other important maturational processes of the neuron. Glutamate receptor expression and exact subunit composition, for example, are unique in each cell population and influence the cells’ selective vulnerability during development (36).
Myelination
Myelin insulates individual axons and greatly increases speed of conduction. It is not essential in all nerves, and many autonomic fibers of the peripheral nervous system remain unmyelinated throughout life. Conduction velocity in central pathways coordinates time-related impulses from different centers that converge on a distant target and ensures that action potentials are not lost by synaptic block. The nervous system functions on the basis of temporal summation of impulses to relay messages across synapses.
Myelination of CNS pathways occurs in a predictable spatial and temporal sequence. Some tracts myelinate as early as 14 weeks’ gestation and complete their cycle in a few weeks. Examples include the spinal roots, medial longitudinal fasciculus, dorsal columns of the spinal cord, and most cranial nerves. Between 22 and 24 weeks’ gestation, myelination progresses in the olivary and cerebellar connections, the ansa lenticularis of the globus pallidus, the sensory trigeminal nerve, the auditory pathways, and the acoustic nerve, as well as in the trapezoid body, lateral lemniscus, and brachium of the inferior colliculus. By contrast, the optic nerve and the geniculocalcarine tract (i.e., optic radiations) do not begin to acquire myelin until near term. Some pathways have myelination cycles measured in years. The corpus callosum begins myelinating at 2 months postnatally, and the cycle is not complete until midadolescence. Some ipsilateral association fibers that connect the frontal with the temporal and parietal lobes do not achieve full myelination until about 32 years of age (69).
Myelination can now be accurately measured in specific central pathways of the living patient with the use of T2-weighted magnetic resonance imaging (MRI) sequences, albeit at a somewhat later time than with traditional myelin stains of brain tissue sections, such as Luxol fast blue. Newer neuropathologic methods that use gallocyanin and immunore activity to myelin basic protein may detect myelination even earlier than the traditional stains in postmortem tissue or in brain biopsy samples. Electron microscopy remains the most sensitive way to demonstrate the earliest myelination in tissue sections. Immunocytochemical markers of oligodendrocyte precursor cells provide another means of studying early myelination in the fetal brain (70).
SPECIAL FEATURES OF CEREBRAL CORTICAL DEVELOPMENT
What Is a Cortex?
Nuclear and cortical types of architecture characterize the vertebrate CNS. Neither pattern corresponds to the architecture of a ganglion in the peripheral nervous system (14). Nuclear organization is most common in the brainstem, most of the thalamus, and in the deep telencephalic nuclei (i.e., basal ganglia). It consists of aggregates of one or two types of neurons that often appear homogeneous and without special histologic arrangement but that actually have a highly defined somatotopic arrangement of synaptic relations. Scattered neurons along tracts, such as the fasciculus solitarius, the diagonal band of Broca, or the bed nucleus of
the stria terminalis, also are regarded as nuclear architecture, even though not all neurons are in compact aggregates with sharply demarcated borders on histologic examination. Neuroanatomic nuclei may contain more than one type of neuron, either mixed together or segregated into clusters. Examples are the magnocellular and parvocellular parts of the red nucleus of the midbrain and the mixed large and small neurons comprising the caudate nucleus.
the stria terminalis, also are regarded as nuclear architecture, even though not all neurons are in compact aggregates with sharply demarcated borders on histologic examination. Neuroanatomic nuclei may contain more than one type of neuron, either mixed together or segregated into clusters. Examples are the magnocellular and parvocellular parts of the red nucleus of the midbrain and the mixed large and small neurons comprising the caudate nucleus.
Cortical organization is a laminated architecture, with layers of neurons generally of the same type forming synaptic circuits with those of other layers. As a result, the synaptic architecture is columnar or perpendicular to the surface, whereas the histologic laminae are horizontal or parallel to the surface. Cortical architecture is just as early an evolutionary development as nuclear architecture, even though the mammalian cerebral neocortex is more recent. Cortical architecture is found in the cerebellar cortex of all vertebrates that possess a cerebellum; in many parts of the visual system, including the optic tectum (superior colliculus) of the midbrain, the lateral geniculate body of the thalamus, and the retina itself; and in the hippocampus or paleocortex, including both the dentate nucleus and the cornu ammonis. Many additional, small, inconspicuous brain regions also exhibit cortical lamination. An example is the islands of Calleja, which lie against the paraolfactory area of the basal forebrain. In these cup-shaped masses of neurons and polymorphic layers of pyramidal cells, fibers enter and leave at the opening of the cup or at the periphery. A cortex is defined, therefore, as a concentric laminar arrangement of neurons forming a band of gray matter with perpendicular columns of synaptic circuitry.
Ontogeny of the Cerebral Neocortex
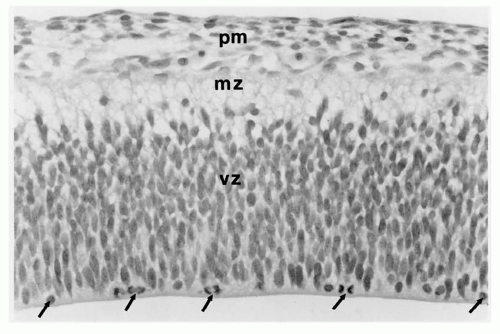
The embryonic prosencephalon or cerebral vesicle “cleaves” sagittally in the midline at 33 days’ gestation to form two symmetric telencephalic hemispheres. In the human fetus at 6 weeks’ gestation, these consist of large ventricles not yet lined by ependyma that are surrounded by two concentric layers: a wide inner layer of neuroepithelium forming the ventricle wall, the ventricular zone, and an outer layer called the marginal zone (Fig. 2.2). The ventricular zone is a pseudostratified columnar epithelium in which every cell has a long cytoplasmic process; the proximal end is attached to the ventricular wall, and the distal end extends to the outer surface of the ventricular zone. The nucleus moves to and from within its own process, with a mitotic phase near the ventricular wall and a resting phase (S-phase) at the junctions with the marginal zone when DNA is being replicated in preparation for another mitosis (71,72).
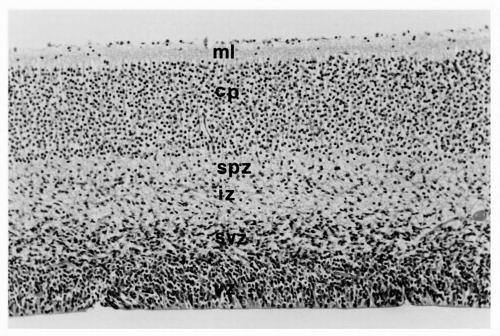
The marginal zone, already a primordial cerebral cortex, has a plexus of bipolar neurons with long processes that extend in either direction parallel to the surface of the hemisphere. By 8 weeks’ gestation, the cerebrum is more complex with more concentric zones (Fig. 2.3). The ventricular zone persists, but peripheral to it is a subventricular zone of postmitotic, premigratory neuroblasts and radial glial cells; these specialized fetal astrocytes are not attached to the ventricular surface but have long processes extending to the pial surface of the brain to guide migratory neuroblasts and later glioblasts to the cortical plate. The next concentric zone is the intermediate zone of migrating neuroblasts and radial glial processes; it will later become the subcortical white matter. The former outermost or marginal zone, or the two-layer cerebrum, is no longer recognized as a single zone because it is divided by a cortical plate within its center as more neurons complete their journey from the subventricular zone. As the cortical plate forms, the outer portion of the marginal zone remains cell sparse, except for scattered Cajal-Retzius neurons, and is now called the molecular layer; the innermost part of the marginal zone beneath the cortical plate becomes the subplate zone. The cerebral cortex at this stage is said to be a four-layer structure, the outer layer being the molecular zone that will become layer 1 of the mature cortex. The second layer is the densely cellular cortical plate that corresponds to layers 2 through 6 of the mature cortex. The third layer is the subplate zone derived from the inner part of the previous marginal zone, and the fourth or deepest layer consists of still migrating neuroblasts within the intermediate zone. Arrest at this stage of development may result in malformations like some polymicrogyria and lissencephaly type 1 that have a four-layer cortex on histologic examination.
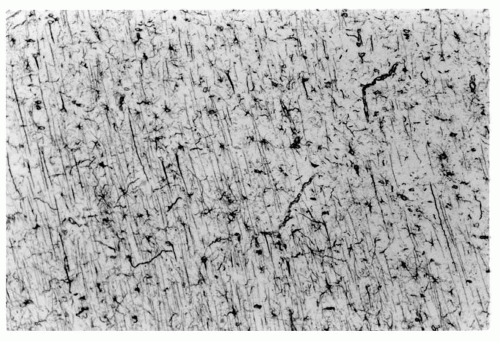
The radial neuroblast migration to the cortical plate occurs in waves and has an inside-out arrangement: the first wave becomes layer 6 because subsequent waves reach the cortical surface, displacing earlier arrivals into deeper positions. Layer 2 thus contains the most recently arrived neuroblasts. Layer 1 remains the oldest layer because it existed before radial migration began. Tangential migration occurs during all stages of radial migration, but particularly in the early stages. Before 22 weeks’ gestation, the cerebral cortex appears histologically to be arranged as vertical columns of neurons rather than as horizontal layers and, indeed, the intracortical synaptic connections are columnar, more between than within layers (Fig. 2.4).
Further histogenesis of the cerebral cortical plate consists of the separation of the tightly packed individual neuroblasts by neuropil. This tissue is derived from the growth of dendritic processes and axons, synapse formation, and the addition of astrocytes migrating from the subventricular zone (i.e., germinal matrix), mostly during the second half of gestation, to become the protoplasmic astrocytes of the mature cerebral cortex. Lamination of the cortical plate is present from the onset, even if not yet evident histologically because of the tightly packed small cortical neurons, but becomes apparent as the neuropil increases and neurons grow (Fig. 2.3). The size of each layer is characteristic of the regional development of the neocortex. In the frontal lobe, the large pyramidal cells of layers 5 and 6 are prominent and numerous, but layer 4 is thin; in the occipital lobe,
layer 4 is most prominent and is even subdivided, whereas the large pyramidal cells of deeper layers are fewer. Radial glial cells are most prominent from 8 to 20 weeks’ gestation and are fewer in the second half of gestation. The radial glial fibers are retracted and their specialized cells become fibrillary astrocytes in the white matter.
layer 4 is most prominent and is even subdivided, whereas the large pyramidal cells of deeper layers are fewer. Radial glial cells are most prominent from 8 to 20 weeks’ gestation and are fewer in the second half of gestation. The radial glial fibers are retracted and their specialized cells become fibrillary astrocytes in the white matter.
More than one type of neuron may appear in a particular layer at times, especially in restricted regions of cortex, and new neuronal types are still being discovered. Large spindle neurons appear after 4 months of age in the anterior cingulate gyrus (Brodmann area 24) and frontoinsular cortex. As much as four times larger than the large pyramidal neurons that populate most of layer 5, these bipolar cells have their axis perpendicular to the surface of the brain, large dendritic trees at either end, and a robust axon hillock. Like the neurons of the islands of Calleja, they exhibit serotonin-B and dopamine-D3 membrane receptors, but their origin and function are unknown (J. Allman, personal communication).
The ganglionic eminence is a densely cellular part of the subventricular zone of the early fetal forebrain that forms part of the deep telencephalic nuclei and contributes some neuroblasts to the cortex by tangential migration (Fig. 2.5).
Cajal-Retzius Neurons
These first mature neurons of the cerebral cortex are already secretory and functional before the first wave of radial migration of neuroblasts from the subventricular zone (73,74). Cajal-Retzius neurons form a plexus in the marginal zone, and, after the cortical plate begins to form, they make the initial intrinsic synaptic circuits of the primordial cerebral cortex, synapsing with neurons of layer 6 and eventually with neurons of all layers (73). Their bipolar long processes, which run parallel to the cortical surface, have numerous collateral axons that branch perpendicular to the main axon and plunge into the cortical plate; however, dendrites growing toward the cortical surface of deep cells also meet Cajal-Retzius axons in synaptic relation. As the cortex grows, Cajal-Retzius neurons become increasingly sparse in the molecular layer because no new ones are produced. They were thought to disappear at maturity by apoptosis, but they persist, albeit so diluted by the expansion of the cortex that only occasional examples are
found in layer 1 of the neonatal brain and even rarer cells in the adult.
found in layer 1 of the neonatal brain and even rarer cells in the adult.
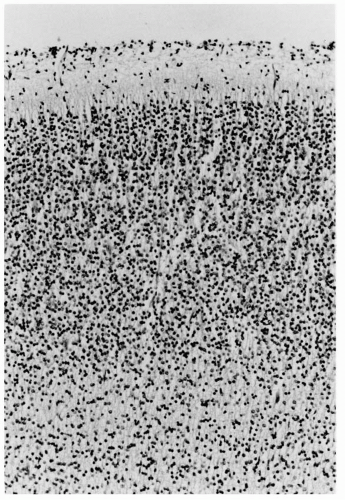 Figure 2.3 Cerebral cortex of a normal 22-week human fetus. Incipient cortical lamination is evident, although not as distinct as at older ages and considerable neuropil separates individual neurons (compare with the cortical plate in Fig. 2.2). This neuropil is mostly dentritic processes, axons, and some glial cells and their processes. Neurons of the cortex have more cytoplasm, and larger pyramidal cells of layers 3, 5, and 6 are distinguished from the smaller, rounder granule cells of layers 2 and 4. The cortical architecture is histologically transitional from a vertically columnar to a horizontally laminar appearance (hematoxylin and eosin stain; original magnification ×250). |
Cajal-Retzius neurons produce GABA, the first neurons of the cerebral cortex to do so. They also cosynthesize several calcium-binding proteins that act as secondary transmitters, including calbindin, parvalbumin, and possibly calretinin. Because neurons arising in the ganglionic eminence are GABAergic, this site has been proposed as the origin of these neurons of the marginal zone. The mesencephalic neuromere is another strong candidate site of origin, but where these early neurons actually come from remains uncertain. It is unlikely that they originate in situ from the molecular zone of the telencephalon, and they are not part of even the earliest radial migration from the subventricular zone.
Beyond helping to establish early intracortical synaptic circuitry and being an intrinsic part of the cortex, Cajal-Retzius neurons express several genes important in corticogenesis, including Reelin (RLN), LIS1, EMX2, and DS-CAM. These neurons also may have a role in cortical repair, particularly in fetal life and early infancy (75).
Subplate Neurons
Located deep in the marginal zone, these neurons are present before the first wave of radial neuroblast migration, similar to Cajal-Retzius neurons of the superficial aspect of the marginal zone (73). With maturation of the cerebral cortex, subplate neurons are incorporated into layer 6 and participate in mature synaptic networks within the cortex (76), but the subplate zone is a transitory fetal feature not present at term, unlike the molecular layer at the surface (77). The subplate zone at midgestation is cell sparse relative to the densely cellular cortical plate. Subplate neurons project descending pioneer axons to form the initial internal capsule as a template for the large pyramidal cells of the deep cortical plate layers, whose long axons project through this provisional capsule as the permanent corticobulbar and corticospinal tracts (78). They also form the earliest commissural connections of the hippocampi.
Subplate neurons are involved in the formation of areaspecific thalamocortical connections (79) and, by modulating BDNF and thalamocortical connectivity, of ocular dominance columns of the striate cortex (80,81). Subplate neuronal ablation in animals disrupts functional maturation in the visual cortical columns, including ocular dominance and orientation selectivity (80,82,83).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree