Chapter 19 Basal Ganglia
In 1817 James Parkinson, an English country physician, published a brief monograph entitled An Essay on the Shaking Palsy, in which he described the symptoms of several individuals who had the disease that now bears his name. Parkinsonian patients, as described in more detail later in this chapter, are characterized by tremor, generally increased muscle tone, and difficulty initiating voluntary movements, which are unusually small and slow once begun. This and related disorders, whose signs typically include some combination of slowed or diminished movements, involuntary movements, and generalized alterations in muscle tone, have come to be associated with damage to the basal ganglia. They were long referred to as extrapyramidal disorders to distinguish them from disorders involving the corticospinal (pyramidal) system. This terminology is no longer used because, as discussed later in this chapter, the two systems are ultimately interdigitated; for example, many of the involuntary movements following basal ganglia damage are actually effected through the corticospinal tract.
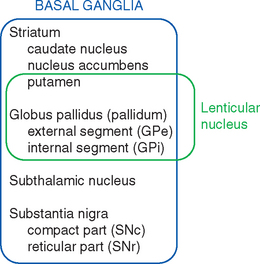
The Basal Ganglia Include Five Major Nuclei
The meaning of the term basal ganglia has evolved over time, but it is now used to refer to those structures whose damage causes the distinctive kinds of movement disorders (and other disorders) described in this chapter. These structures include some large subcortical nuclei of each cerebral hemisphere—the putamen, caudate nucleus, nucleus accumbens, and globus pallidus—together with the diencephalic subthalamic nucleus and the substantia nigra of the rostral midbrain (Figs. 19-1 and 19-2).
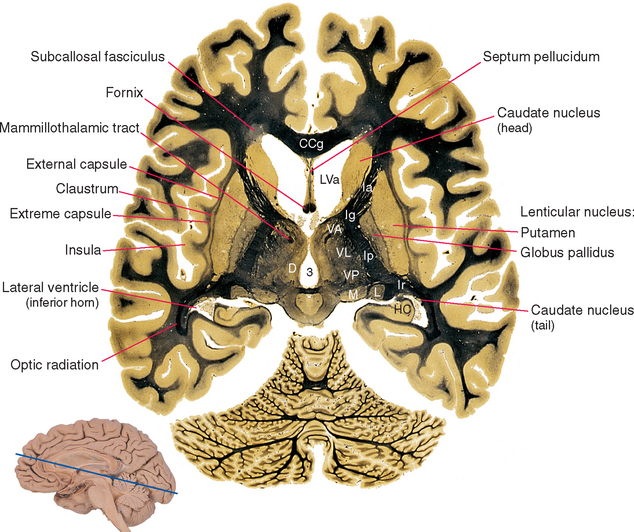
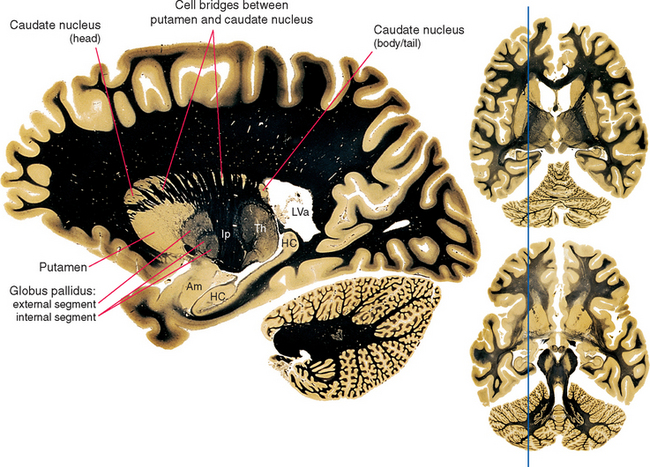
Figure 19-1 Basal ganglia and surrounding structures, as seen in an axial section. The claustrum is a plate of gray matter with a largely unknown function, layered between the putamen and the insular cortex. The external capsule, between the claustrum and the putamen, is a route through which many projections from the cerebral cortex reach the putamen; many cortical fibers reach the caudate nucleus through the subcallosal fasciculus. The extreme capsule houses association fibers that interconnect different cortical areas (see Chapter 22), third ventricle; CCg, genu of the corpus callosum; D, dorsomedial nucleus; HC, hippocampus; Ia, Ig, Ip, and Ir, internal capsule—anterior limb, genu, posterior limb, and retrolenticular part; L, lateral geniculate nucleus; LVa, anterior horn of the lateral ventricle; M, medial geniculate nucleus; VA, VL, and VP, ventral anterior, ventral lateral, and ventral posterior nuclei.
(Adapted from Nolte J, Angevine JB Jr: The human brain in photographs and diagrams, ed 3, St. Louis, 2007, Mosby.)
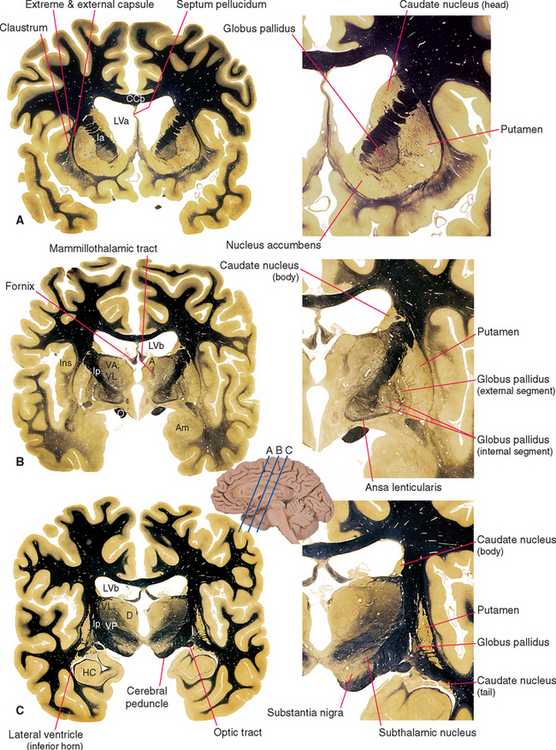
Figure 19-2 Basal ganglia and surrounding structures, as seen in coronal sections. The ansa lenticularis is an output bundle leaving the globus pallidus (see Fig. 19-15). A, anterior nucleus (of the thalamus); Am, amygdala; CCb, body of the corpus callosum; D, dorsomedial nucleus; HC, hippocampus; Ia and Ip, internal capsule—anterior limb and posterior limb; Ins, insula; LVa and LVb, anterior horn and body of the lateral ventricle; O, optic tract; VA, VL, and VP, ventral anterior, ventral lateral, and ventral posterior nuclei.
(Adapted from Nolte J, Angevine JB Jr: The human brain in photographs and diagrams, ed 3, St. Louis, 2007, Mosby.)
Various names are applied to different combinations of members of the basal ganglia (Fig. 19-3). The putamen, caudate nucleus, and nucleus accumbens have a common embryological origin, identical histological appearances, and similar connections. One indication of this common origin is their physical continuity just above the orbital surface of the frontal lobe, where the head of the caudate merges with nucleus accumbens,* which in turn merges with the anterior part of the putamen. Another indication is the bridges of gray matter growing across the internal capsule between the putamen and the caudate nucleus, giving this region a striped appearance in many planes of section (Fig. 19-4). Because of this appearance, the putamen, caudate nucleus, and nucleus accumbens together are referred to as the striatum. The putamen and globus pallidus have different connections but are physically apposed, and together they are referred to as the lenticular or lentiform nucleus (from the Latin word for “lentil”).
The Striatum and Globus Pallidus Are the Major Forebrain Components of the Basal Ganglia
The lenticular nucleus is shaped somewhat like a wedge cut from a sphere (Figs. 19-1 and 19-2). The putamen (from the Latin for “husk”), which is approximately coextensive with the insula, forms the outermost portion of this wedge. It is separated from the more medial globus pallidus by a thin lateral medullary lamina of myelinated fibers. The globus pallidus is itself divided into internal and external portions by a medial medullary lamina. In unstained sections through the lenticular nucleus, the globus pallidus has a distinctively pale appearance as a result of the large number of myelinated fibers that traverse it, terminate in it, and originate in it. (In myelin-stained sections such as those shown in Figures 19-1 and 19-2, it is therefore relatively dark.)
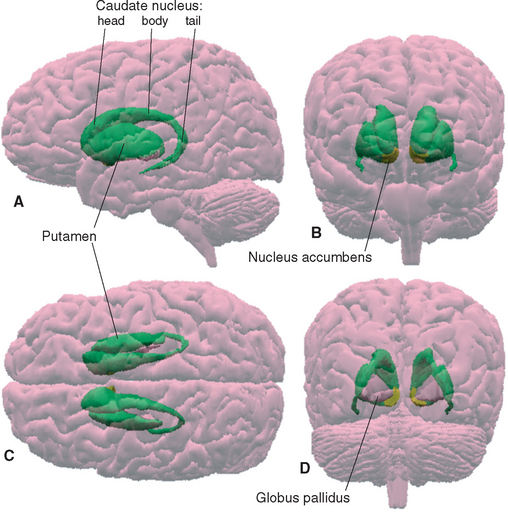
The putamen starts out at the site where the diencephalon and telencephalon fuse during development (see Figs. 2-12 and 2-13), and it stays in this location. The caudate nucleus, in contrast, remains in the wall of the lateral ventricle and grows with it in a C-shaped course. The result is that the caudate nucleus (Latin for “nucleus with a tail”) winds up with an enlarged head that bulges into the anterior horn, a body in the lateral wall of the body of the ventricle, and a slender tail that borders on the inferior horn (Figs. 19-1, 19-2, and 19-5). In the temporal lobe, the tail of the caudate nucleus is continuous with the amygdala, which in turn is continuous with the putamen (see Fig. 25-5), but these physical continuities are of no apparent functional significance.
The Subthalamic Nucleus and Substantia Nigra Are Interconnected with the Striatum and Globus Pallidus
The subthalamic nucleus, shaped like a biconvex lens enveloped in white matter, is located between the medial part of the cerebral peduncle and the thalamus (Fig. 19-2C). Although relatively small, it has major interconnections with other parts of the basal ganglia (see Fig. 19-17).
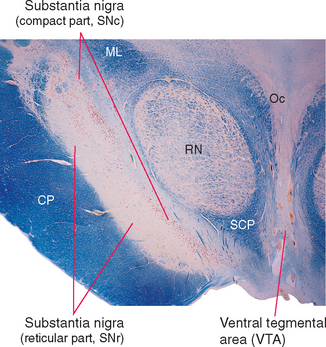
The region referred to as the substantia nigra actually has two parts (Fig. 19-6)—a dorsal compact part (SNc) that contains closely packed, pigmented neurons, and a reticular part (SNr) nearer the cerebral peduncle that contains more loosely packed neurons, most of which are nonpigmented. These correspond to the two distinctly different ways in which the substantia nigra participates in the circuitry of the basal ganglia: the compact part provides widespread, modulatory, dopaminergic projections to other parts of the basal ganglia, whereas the reticular part is one of the basal ganglia output nuclei (see Figs. 19-7 and 19-9).
Basal Ganglia Circuitry Involves Multiple Parallel Loops That Modulate Cortical Output
Afferents from the Cortex Reach the Striatum and Subthalamic Nucleus; Efferents Leave from the Globus Pallidus and Substantia Nigra
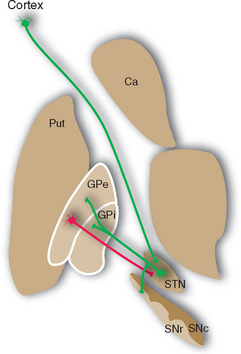
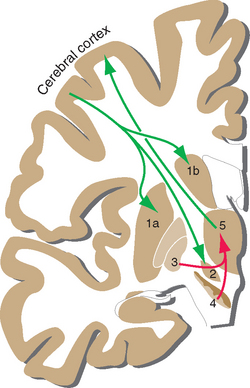
Projections from the cerebral cortex reach the striatum and subthalamic nucleus (Fig. 19-7), which in this sense are the principal input elements of the basal ganglia. Outputs leave from the internal segment of the globus pallidus (GPi) and the SNr. The links between these input and output structures are discussed a little later in this chapter. The corticostriate inputs and the projections back to the cortex from the thalamus all make excitatory (glutamate) connections, whereas the GPi-SNr outputs are all inhibitory (γ-aminobutyric acid [GABA]). There are multiple versions of this loop, all similar in principle but each utilizing different cortical areas and a distinctive portion of the striatum, globus pallidus, and other nuclei. It was once thought that each included a frontal or limbic area among the cortical input areas and that each returned to a frontal or limbic area. However, more recent work indicates that some loops begin and end in parietal or temporal association cortex, so interactions with the basal ganglia may characterize most cortical areas.
The major circuit through which the basal ganglia participate in the control of movement provides one example of such a loop. The striatum and globus pallidus form by far the largest part of the basal ganglia, yet they have no way to affect motor neurons directly (see Figs. 18-7 and 18-8). Thus the only way the basal ganglia can play a role in the control of movement is by somehow influencing one or more of the descending pathways mentioned in the previous chapter. They do so primarily by affecting the activity of motor areas of the cerebral cortex (and, to a degree, through projections to the reticular formation). Somatosensory and motor areas project to a portion of the striatum (mostly putamen), which in turn projects by way of GPi to the ventral anterior and ventral lateral (VA/VL) nuclei; the circuit is completed by projections from VA/VL back to motor areas of the cortex. Other basal ganglia loops use their own distinctive portions of the striatum, subthalamic nucleus, globus pallidus, substantia nigra, thalamus, and cerebral cortex. Collectively the loops roughly correspond to the putamen, caudate nucleus, and nucleus accumbens, which receive inputs from motor and somatosensory cortex, association cortex, and limbic areas, respectively (Fig. 19-8). The partitioning of connections among striatal subdivisions is not absolute. For example, limbic inputs reach not only nucleus accumbens but also adjoining parts of the putamen and caudate nucleus. For this reason, all these limbic-recipient regions are recognized as a separate striatal division called the ventral striatum.
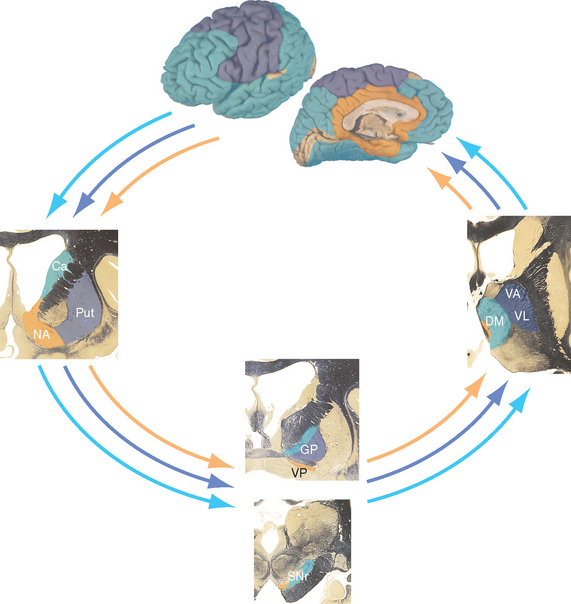
Figure 19-8 Parallel loops through the basal ganglia. In general, projections from association cortex emphasize the caudate nucleus, those from sensorimotor cortex emphasize the putamen, and those from limbic areas emphasize nucleus accumbens. However, the demarcations are not as absolute as this figure implies. For example, the frontal eye field (see Chapter 21) projects to the caudate nucleus, which is involved in eye movement control, and parietal association cortex projects to parts of the putamen as well as to parts of the caudate nucleus. Ca, caudate nucleus; DM, dorsomedial nucleus; GP, globus pallidus; NA, nucleus accumbens; Put, putamen; SNr, substantia nigra (reticular part); VA, ventral anterior nucleus; VL, ventral lateral nucleus; VP, ventral pallidum (a small extension of the globus pallidus under the anterior commissure).
Interconnections of the Basal Ganglia Determine the Pattern of Their Outputs
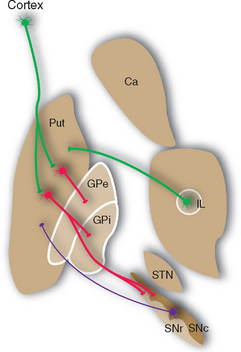
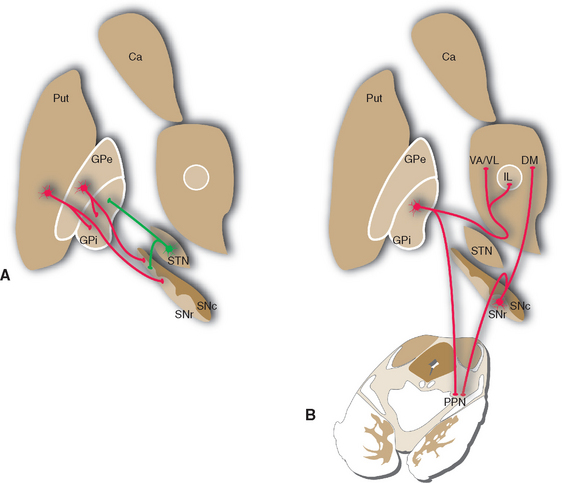
Most of the remaining connections of the basal ganglia fall into four categories: widespread dopaminergic projections from the compact part of the substantia nigra to other parts of the basal ganglia, especially the striatum; multiple inhibitory interconnections between different parts of the basal ganglia (see Fig. 19-13); excitatory projections from the subthalamic nucleus to the globus pallidus (see Fig. 19-17); and interconnections of thalamic intralaminar nuclei with the striatum and globus pallidus (see Figs. 19-9 and 19-14). A striking feature of these connections is the large proportion of inhibitory synapses. All the outputs from the striatum, globus pallidus, and reticular part of the substantia nigra are inhibitory, using GABA as a transmitter. The only prominent source of excitatory (glutamate) projections is the subthalamic nucleus.
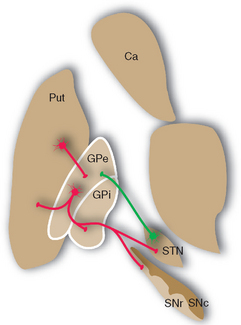
Figure 19-13 Major connections of the external segment of the globus pallidus (GPe); only striatal inputs from the putamen (Put) are shown, although the caudate nucleus (Ca) and ventral striatum are involved in similar ways. For simplicity, all the striatal projections to the internal segment of the globus pallidus (Gpi) and the reticular part of the substantia nigra (SNr) are shown as coming from the putamen. Most of those to SNr actually come from the caudate nucleus, and the ventral striatum projects to the ventral pallidum (see Fig. 23-22) and SNr. Excitatory connections are shown in green, inhibitory connections in red. SNc, compact part of the substantia nigra; STN, subthalamic nucleus.
The Cerebral Cortex, Substantia Nigra, and Thalamus Project to the Striatum
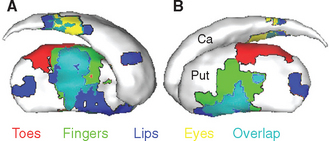
The caudate nucleus, putamen, and ventral striatum receive inputs from the cerebral cortex, the substantia nigra (SNc) and nearby ventral tegmental area, and the intralaminar nuclei of the thalamus (Fig. 19-9). The cortical input is by far the most massive of the three. These fibers originate in most areas of the cortex, pass through the internal and external capsules, and end in a roughly topographical pattern in the striatum. The putamen underlies the insula, at the base of the central sulcus, and receives most of the projections from the motor and somatosensory cortex; not surprisingly, body parts are mapped out systematically in the putamen (Fig. 19-10). The caudate nucleus, as it curves around with the ventricular system, receives most of the projections from association areas; as the size and location of the head of the caudate might imply, the projection is particularly heavy from prefrontal cortex. Finally, the ventral striatum receives inputs not only from limbic cortex but also from the hippocampus and amygdala. Through these inputs, the basal ganglia are constantly informed about most aspects of cortical function. The compact part of the substantia nigra and the ventral tegmental area project systematically to all areas of the striatum (and to other parts of the basal ganglia) by way of very fine axons that use dopamine as their neurotransmitter. Destruction of this nigrostriatal pathway is the major factor causing Parkinson’s disease (see Fig. 19-20). Finally, the intralaminar nuclei, especially the centromedian and parafascicular nuclei, project to the striatum. Many of these same fibers, as mentioned in Chapter 16, have collateral branches that end in the cerebral cortex. This thalamostriate pathway is particularly well developed in primates, but very little is known of its normal function. However, abnormal activity in this intralaminar nuclei→basal ganglia→intralaminar nuclei loop has been implicated in some basal ganglia disorders.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree