Chapter 6 Blood Supply of the Brain
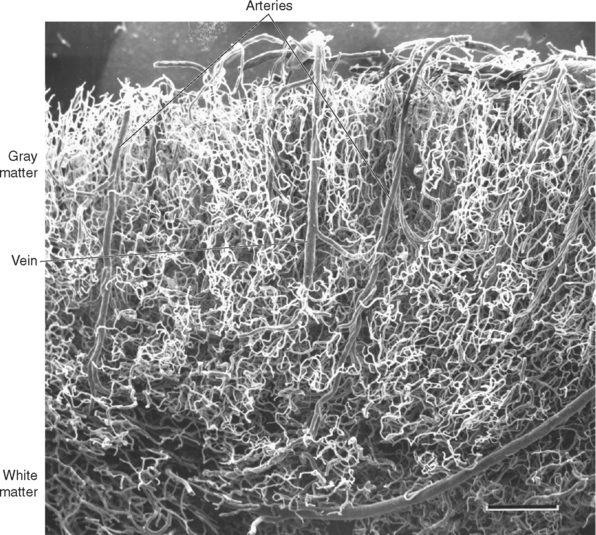
Turtles can walk around for hours with no oxygen supply to their brains. In contrast, our brains are absolutely dependent on a continuous supply of well-oxygenated blood. After just 10 seconds of brain ischemia, we lose consciousness. After 20 seconds, electrical activity ceases, and after just a few minutes, irreversible damage usually begins. Corresponding to this metabolic dependence, blood vessels in the central nervous system (CNS), particularly in gray matter, are arranged in a dense meshwork (Fig. 6-1). An understanding of the brain’s blood supply is essential to an understanding of its normal function and of the consequences of cerebrovascular disease. This chapter provides a general overview of the circulatory system of the CNS. Many subsequent chapters include a more detailed discussion of the arterial supply of individual portions of the CNS.
The Internal Carotid Arteries and Vertebral Arteries Supply the Brain
The arterial supply of the brain and much of the spinal cord is derived from two pairs of vessels, the internal carotid arteries and the vertebral arteries (Fig. 6-2). The internal carotid arteries provide about 80%, supplying most of the telencephalon and much of the diencephalon. The vertebral system provides the remaining 20%, supplying the brainstem and cerebellum, as well as parts of the diencephalon, spinal cord, and occipital and temporal lobes (Table 6-1).
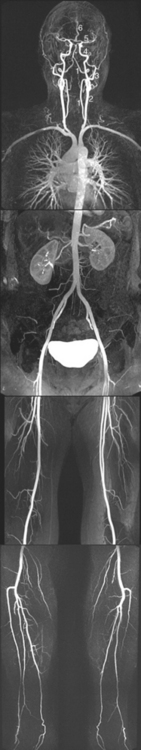
Figure 6-2 Origins of the arterial supply of the brain, seen in a remarkable contrast-enhanced whole-body magnetic resonance angiogram (see Box 6-1) of a healthy 56-year-old man. The vertebral (1) and common carotid (2) arteries can be seen ascending through the neck. The two vertebral arteries fuse to form the basilar artery (*), and each common carotid bifurcates into an external (3) and internal (4) carotid artery. Each internal carotid then ends at the base of the brain by dividing into a middle (5) and anterior (6) cerebral artery.
(From Nael K et al: Radiology 242:865, 2007.)
Table 6-1 Arterial Supply of the Central Nervous System*
| Anatomical Area | Artery |
|---|---|
| Cerebral Hemisphere | |
| Cortical areas | |
| Frontal lobe | |
| Lateral surface | MCA |
| Medial surface | ACA |
| Inferior surface | ACA, MCA |
| Parietal lobe | |
| Lateral surface | MCA |
| Medial surface | ACA |
| Occipital lobe | |
| Lateral surface | MCA† |
| Medial, inferior surfaces | PCA |
| Temporal lobe | |
| Lateral surface | MCA |
| Medial, inferior surfaces | PCA |
| Temporal pole | MCA |
| Limbic lobe | |
| Cingulate gyrus | ACA |
| Parahippocampal gyrus | PCA |
| Insula | MCA |
| Basal ganglia | |
| Caudate nucleus (head) | ACAp, MCAp |
| Putamen | MCAp, ACAp |
| Globus pallidus | AChA, MCAp |
| Limbic structures | |
| Amygdala | AchA |
| Hippocampus | PCA, AchA |
| Internal capsule | MCAp, AChA, ACAp, ICAp |
| Corpus callosum | |
| Genu, body | ACA |
| Splenium | ACA, PCA |
| Diencephalon | |
| Thalamus | PCAp, PComp, AChA |
| Hypothalamus | PComp, ICAp, Acomp |
| Cerebellum | |
| Superior surface | SCA |
| Inferior, anterior surfaces | PICA, AICA |
| Brainstem | |
| Midbrain | PCA, SCA, BA |
| Pons | BA, AICA |
| Medulla | VA, PICA |
| Spinal Cord | |
| Anterior two thirds | ASpA |
| Posterior third | PspA |
ACA, anterior cerebral artery; AChA, anterior choroidal artery; ACom, anterior communicating artery; AICA, anterior inferior cerebellar artery; ASpA, anterior spinal artery; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; p, perforating branches of the artery; PCA, posterior cerebral artery; PCom, posterior communicating artery; PICA, posterior inferior cerebellar artery; PSpA, posterior spinal artery; SCA, superior cerebellar artery; VA, vertebral artery.
* Includes major arteries only; does not include areas of overlap such as ACA-MCA overlap on the lateral surface near the longitudinal fissure. For a pictorial representation, see Figure 6-22. More details on particular areas of supply are included in subsequent chapters.
† Middle cerebral and posterior cerebral territories overlap at the occipital pole. This has important implications for the kinds of visual deficits that follow strokes involving a posterior cerebral artery (see Fig. 17-33).
The Internal Carotid Arteries Supply Most of the Cerebrum
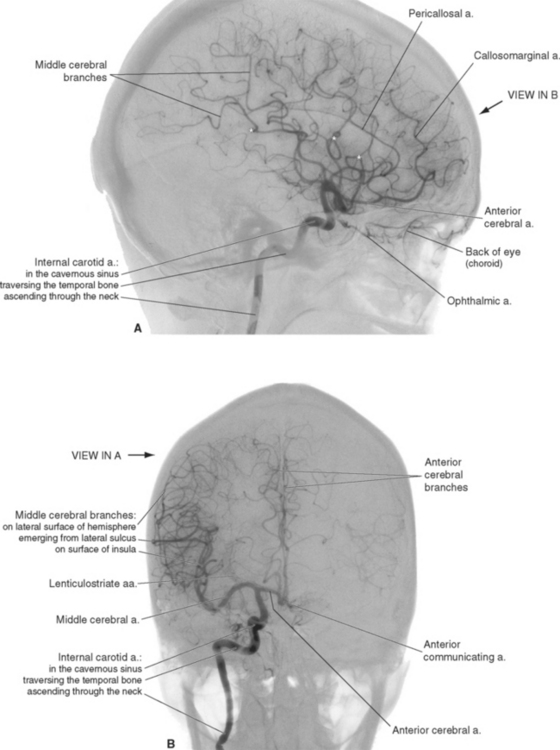
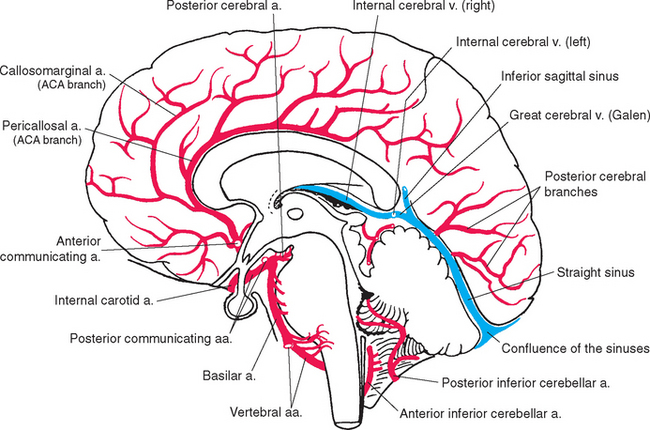
An internal carotid artery ascends through each side of the neck, traverses the petrous temporal bone, passes through the cavernous sinus, and finally reaches the subarachnoid space at the base of the brain (see Fig. 6-15). Just as it leaves the cavernous sinus, it gives rise to the ophthalmic artery, which travels along the optic nerve to the orbit, where it supplies the eye, other orbital contents, and some nearby structures. The internal carotid artery then proceeds superiorly alongside the optic chiasm (Fig. 6-3) and bifurcates into the middle and anterior cerebral arteries. Before bifurcating, it gives rise to two smaller branches, the anterior choroidal artery and the posterior communicating artery. The anterior choroidal artery is a long, thin artery that can be significant clinically because it supplies a number of different structures and is not infrequently involved in cerebrovascular accidents. Along its course (see Fig. 6-6) it supplies the optic tract; the choroid plexus of the inferior horn of the lateral ventricle; some deep structures such as portions of the internal capsule, thalamus, and hippocampus (see Fig. 6-22); and sometimes part of the cerebral peduncle. The posterior communicating artery passes posteriorly, inferior to the optic tract and toward the cerebral peduncle, and joins the posterior cerebral artery (part of the vertebral artery system).
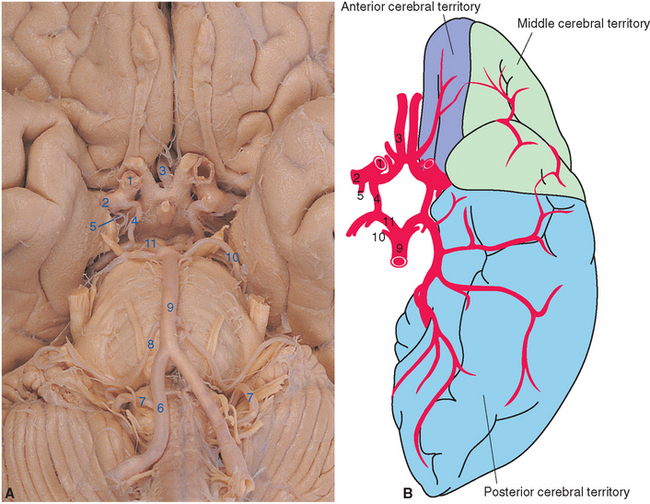
Figure 6-3 Arteries on the inferior surface of the brain (A) and sources of arterial supply to cortical areas (B). The internal carotid artery (1) divides into the middle (2) and anterior (3) cerebral arteries after giving rise to the posterior communicating (4) and anterior choroidal (5) arteries. Collectively, these vessels supply anterior and lateral parts of the cerebrum. The vertebral arteries (6) join to form the basilar artery (9) after giving rise to the posterior inferior cerebellar artery (7). The basilar artery in turn gives rise to the anterior inferior (8) and superior (10) cerebellar arteries before bifurcating into the posterior cerebral arteries (11). Collectively, the vertebral-basilar system supplies the brainstem, much of the diencephalon, and inferior and posterior parts of the cerebral hemispheres. The apparently very large left posterior communicating artery in this specimen is actually a relatively common variant in which the posterior cerebral artery on one side originates from the internal carotid artery instead of the basilar artery (see Fig. 6-12).
(A, from Nolte J, Angevine JB Jr: The human brain in photographs and diagrams, ed 3, St. Louis, 2007, Mosby; dissection courtesy Grant Dahmer, University of Arizona College of Medicine. B, modified from Mettler FA: Neuroanatomy, ed 2, St. Louis, 1948, Mosby.)
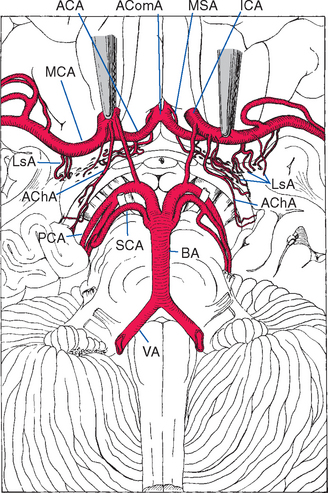
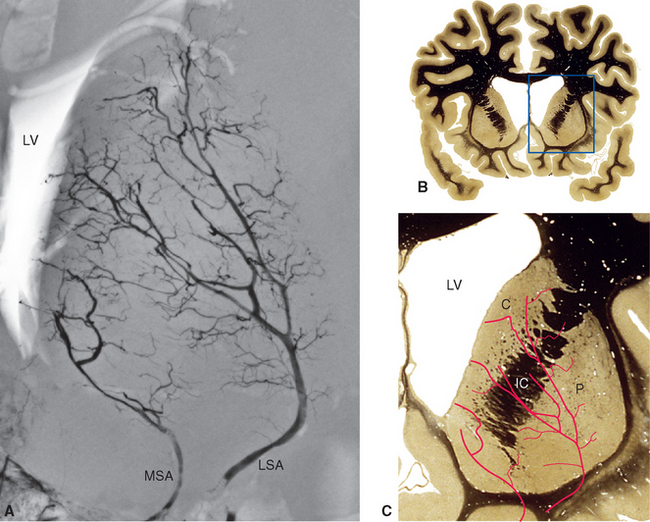
Figure 6-6 Lenticulostriate arteries (LsA), together with perforating branches of the anterior cerebral artery (ACA) and anterior choroidal artery (AChA), entering the anterior perforated substance. Similar perforating branches arise from other arteries of the circle of Willis and from the basilar artery (BA), but these are not included in this drawing. The medial striate artery (MSA), also called the recurrent artery (of Heubner) because of the way it runs back along the anterior cerebral artery, is a major perforating branch important for the supply of the basal ganglia (see Fig. 6-8). AComA, anterior communicating artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; SCA, superior cerebellar artery; VA, vertebral artery.
(From Alexander L: Res Pub Assoc Res Nerv Ment Dis 21:77, 1942.)
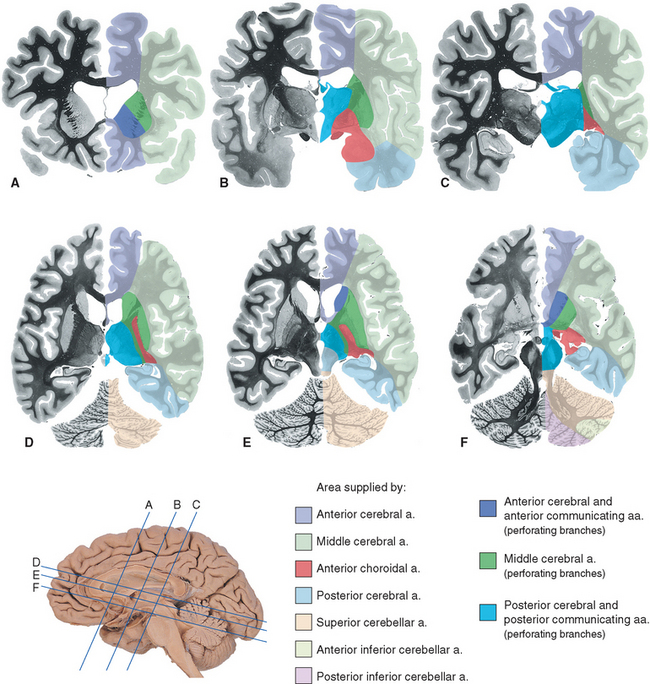
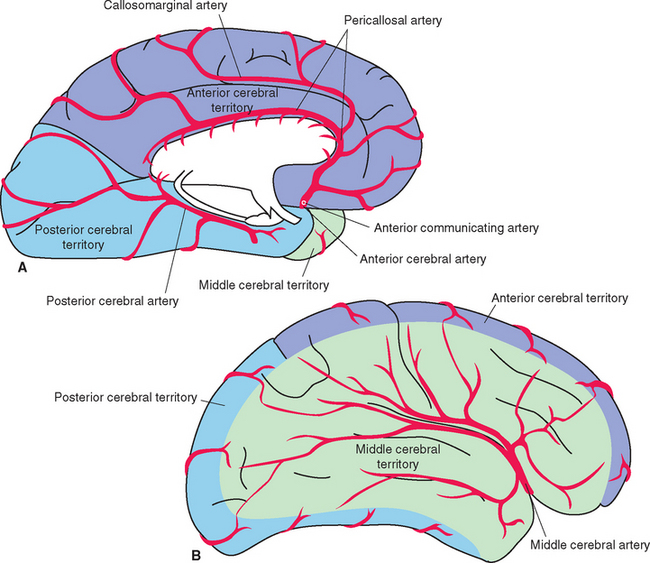
Figure 6-22 Areas of the cerebrum and cerebellum supplied by major arteries and their perforating branches, shown in coronal (A to C) and axial (D to F) sections. Details of the spinal cord and brainstem supply are provided in Chapters 10 and 11.
The anterior cerebral artery runs medially, superior to the optic nerve, and enters the longitudinal fissure (Fig. 6-3). Its branches then arch posteriorly, following the corpus callosum, to supply medial parts of the frontal and parietal lobes (Fig. 6-4A). Some of the smaller branches extend onto the dorsolateral surface of the hemisphere (Fig. 6-4B). The two anterior cerebral arteries, near their entrance into the longitudinal fissure, are connected by the anterior communicating artery (see Figs. 6-6 and 6-11). Distal to the anterior communicating artery, the anterior cerebral artery continues as the pericallosal artery, which stays immediately adjacent to the corpus callosum. Near the genu of the corpus callosum, the callosomarginal artery typically branches off from the pericallosal artery and follows the cingulate sulcus (Fig. 6-4A). Parts of the precentral and postcentral gyri extend onto the medial surface of the frontal and parietal lobes, so occlusion of an anterior cerebral artery causes restricted contralateral motor and somatosensory deficits (affecting the leg more than other parts of the body, because of the somatotopic arrangement shown in Fig. 3-30).
The large middle cerebral artery proceeds laterally into the lateral sulcus (Fig. 6-3). It divides into a number of branches that supply the insula, emerge from the lateral sulcus, and spread out to supply most of the lateral surface of the cerebral hemisphere (Figs. 6-4B and 6-5). Most of the precentral and postcentral gyri are within this area of supply, so occlusion of a middle cerebral artery causes major motor and somatosensory deficits. In addition, if the left hemisphere is the one involved, language deficits are almost invariably found.
Small Perforating Arteries Supply Deep Cerebral Structures
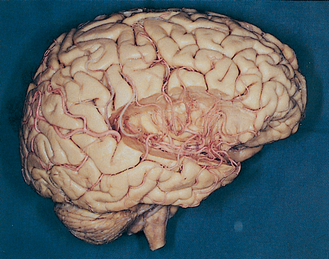
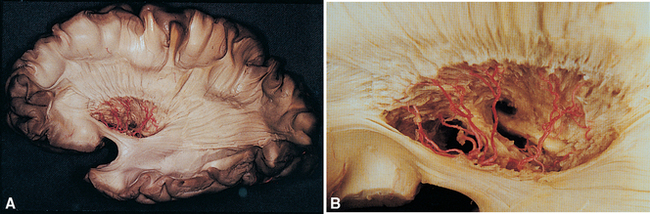
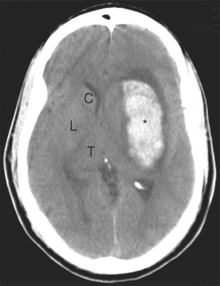
Along its course toward the lateral sulcus, the middle cerebral artery gives rise to up to a dozen very small branches that penetrate the brain near their origin and supply deep structures of the diencephalon and telencephalon (Figs. 6-6 to 6-8). These particular arteries are called the lenticulostriate arteries, but similar small branches, referred to collectively as perforating (or ganglionic) arteries, arise from all the arteries around the base of the brain. Perforating arteries are particularly numerous in the area adjacent to the optic chiasm and in the area between the cerebral peduncles; for this reason they are called the anterior and posterior perforated substances, respectively. The narrow, thin-walled vessels of the anterior perforated substance are involved frequently in strokes (Fig. 6-9). The deep cerebral structures they supply are such that damage to these small vessels can cause neurological deficits out of proportion to their size. For example, the somatosensory projection from the thalamus to the postcentral gyrus must pass through the internal capsule; damage to a small part of the internal capsule from rupture or occlusion of a perforating artery can cause deficits similar to those resulting from damage to a large expanse of cortex.
The Vertebral-Basilar System Supplies the Brainstem and Parts of the Cerebrum and Spinal Cord
The two vertebral arteries run rostrally alongside the medulla and fuse at the junction between the medulla and pons to form the midline basilar artery, which proceeds rostrally along the anterior surface of the pons (Fig. 6-3).
Before joining the basilar artery, each vertebral artery gives rise to three branches: the posterior spinal artery, anterior spinal artery, and posterior inferior cerebellar artery. The posterior spinal artery runs caudally along the posterolateral aspect of the spinal cord and supplies the posterior third of that half of the cord. The anterior spinal artery joins its counterpart from the opposite side, forming a single anterior spinal artery that runs caudally along the anterior midline of the spinal cord, supplying the anterior two thirds of the cord. These small spinal arteries cannot carry enough blood from the vertebral arteries to supply more than the cervical segments of the spinal cord and must be refilled at various points caudal to this (discussed in Chapter 10). The posterior inferior cerebellar artery (often referred to by the acronym PICA), as its name implies, supplies much of the inferior surface of the cerebellar hemisphere (Fig. 6-10); however, it sends branches to other structures on its way to the cerebellum. As it curves around the brainstem, the PICA supplies much of the lateral medulla, as well as the choroid plexus of the fourth ventricle. This is a uniform occurrence in the large named branches of the vertebral-basilar system, directly comparable to the perforating branches from vessels such as the middle cerebral artery: on their way to their major area of supply, arteries in the vertebral-basilar system send branches to brainstem structures. By knowing the brainstem level at which these large named branches emerge, one can make reasonably accurate inferences about the blood supply of any given region of the brainstem (see Figs. 11-29 and 11-30).
The posterior cerebral artery curves around the midbrain and passes through the superior cistern; its branches spread out to supply the medial and inferior surfaces of the occipital and temporal lobes (Figs. 6-3, 6-4A, and 6-10). Along the way, it sends branches to the rostral midbrain and posterior parts of the diencephalon. It also gives rise to several posterior choroidal arteries, which supply the choroid plexus of the third ventricle and the body of the lateral ventricle. The anterior and posterior choroidal arteries form anastomoses in the vicinity of the glomus. The primary visual cortex is located in the occipital lobe, so occlusion of a posterior cerebral artery at its origin leads to visual field losses in addition to other deficits referable to the midbrain and diencephalon.
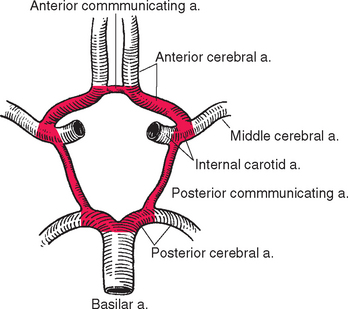
The Circle of Willis Interconnects the Internal Carotid and Vertebral-Basilar Systems
The posterior cerebral artery is connected to the internal carotid artery by the posterior communicating artery. This completes an arterial polygon called the circle of Willis (Figs. 6-3 and 6-11), through which the anterior cerebral, internal carotid, and posterior cerebral arteries of both sides are interconnected. Normally, little blood flows around this circle because the appropriate pressure differentials are not present: the arterial pressure in the internal carotid arteries is about the same as that in the posterior cerebral arteries, so little blood flows through the posterior communicating arteries. However, if one major vessel becomes occluded, either within the circle of Willis or proximal to it, the communicating arteries may allow critically important anastomotic flow and prevent neurological damage. By such a mechanism it would be theoretically possible (though highly unlikely) for the entire brain to be perfused by just one of the four major arteries that normally supply it. The anterior and posterior communicating arteries are quite variable in size (see later), so the establishment of effective anastomotic flow in the event of an arterial occlusion may also depend on the time course of the occlusion. A small communicating artery can enlarge slowly to compensate for a slowly developing occlusion, but in such a case an abrupt blockage might cause serious damage.
Designating the circle of Willis shown in Figure 6-11 as “normal” is largely a nod to an aesthetic need, because fewer than half the circles have this appearance. Some frequently seen “abnormalities” are indicated in Figure 6-12. Asymmetries are common (as in the brain shown in Fig. 6-3A): one or more of the communicating arteries may be very small (hypoplastic) or absent;* one anterior cerebral artery may be much smaller at its origin than the other; one posterior cerebral artery may retain its embryological origin from the internal carotid and be connected to the basilar artery through a posterior communicating artery. These asymmetries in the circle of Willis lead to corresponding asymmetries in the way the brain is supplied (Fig. 6-13).
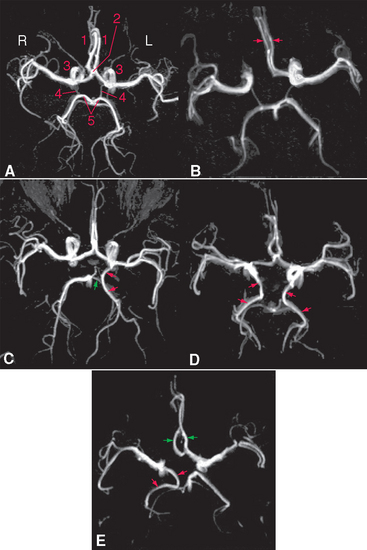
Figure 6-12 Variants of the circle of Willis, demonstrated by magnetic resonance angiography (see Box 6-1). A, Normal circle: 1, anterior cerebral artery; 2, anterior communicating artery; 3, internal carotid artery; 4, posterior communicating artery; 5, posterior cerebral artery. B, Circle with a hypoplastic initial segment of the right anterior cerebral artery; both anterior cerebral arteries (red arrows) arise from the left internal carotid. This occurs in 5% to 10% of the general population. C, The left posterior cerebral artery (red arrows) arises from the internal carotid, and the posterior communicating artery (green arrow) connects it to the basilar artery and the other posterior cerebral artery. This occurs in about 20% of the population. D, Both posterior cerebral arteries (red arrows) arise from the internal carotids. This variation is found about 5% of the time. E, Both anterior cerebral arteries (green arrows) arise from the left internal carotid, and the right posterior cerebral artery (red arrows) arises from the right internal carotid.
(A to D, from Hendrikse J et al: Radiology 235:184, 2005. E, from van Laar PJ et al: NeuroImage 29:136, 2006.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree