BMPs in Cervical Spine Surgery
Vikas V. Patel
Sergiu Botolin
Hyun Bae
The true era of bone regenerative science began over 40 years ago with Dr. Marshall Urist’s experiments that demonstrated new bone formation by implantation of demineralized bone matrix at extraskeletal host sites (1). In 1984, his group reported successful isolation and purification of bone morphogenetic protein (BMP) from hundreds of kilograms of bovine bone and demonstrated their ability to form large bone deposits in vivo (2). In the early 1990s, multiple members of the BMP family have been identified, and their human cDNA has been cloned allowing mass production of these molecules by recombinant techniques (3).
BONE MORPHOGENETIC PROTEINS
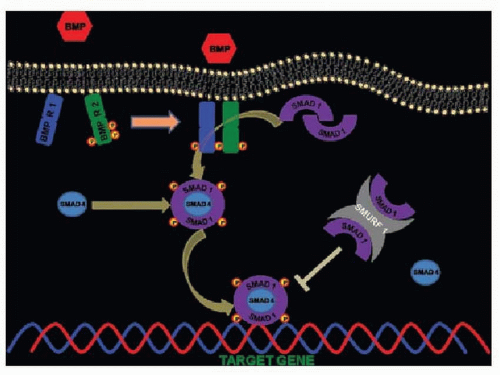
BMPs are secreted growth factors, which, based on amino acid homology of a highly conserved seven-cysteine domain in the carboxy-terminal region of the proteins, form a subgroup of the transforming growth factor-β superfamily (4). They are dimeric proteins with a single interchain disulfide bond. The dimeric conformation is an absolute requirement for the biologic action of BMPs (5). BMP dimers bind to serine/threonine kinase receptors type I and II and upon binding type II receptors transphosphorylate type I receptors, which in turn phosphorylate members of the Smad family of transcription factors (Fig. 117.1). These Smad’s are subsequently translocated to the nucleus, where they activate transcription of target genes (6). Today, the BMP family includes more than 20 members; however, only BMP-2, BMP-3, BMP-4, BMP-6, and BMP-7 have been reported to have osteoinduction activity (7).
CONTEMPORARY STATUS OF BMPS
Out of the five osteoinductive BMPs, only two human recombinant BMPs (hrBMPs) are currently approved by the U.S. Food and Drug Administration (FDA) for use in human surgery. OP-1 (Stryker Corp., Kalamazoo, MI) consists of rhBMP-7 and bovine collagen, which is reconstituted with saline to form a paste or can be formed into a putty with the addition of carboxymethylcellulose. OP-1 has received humanitarian device exemption approval as an alternative to autograft in recalcitrant long bone nonunions (8) and as an alternative to autograft in compromised patients requiring revision dorsolateral (intertransverse) lumbar spinal fusion (9,10). The Infuse system (Medtronic Sofamor Danek, Memphis, TN) consists of rhBMP-2 on an absorbable collagen sponge carrier and has been approved by the FDA for use in anterior interbody lumbar fusion from L2 to S1 in combination with a titanium fusion cage (11), for the treatment of acute open fractures of the tibial shaft (12), and for oral maxillofacial reconstructions (13). Clinical studies focused on OP-1 use as an autograft substitute in lumbar fusion concluded that there was no significant difference between OP-1 and iliac crest grafts and no adverse effects where observed with OP-1 while occasional persistent pain at the graft harvest site was reported for the autograft group (14,15). Use of rhBMP-2 in lumbar spine fusion was also reported to have a positive influence on clinical outcomes with earlier improvement in Oswestry Disability Questionnaire scores and similar fusion rates to the autograft control group (16,17).
BMP’S IN CERVICAL SPINE SURGERY
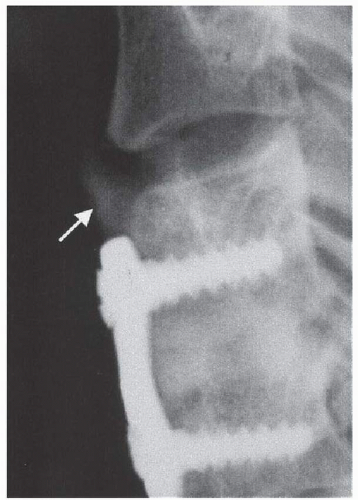
As a consequence of the successful experience with their use in lumbar spine, BMPs soon became a target of interest in cervical fusions. An early prospective, randomized, and controlled FDA-approved pilot clinical trial on 33 patients with rhBMP-2 for ventral cervical fusion reported 100% fusion rates in both BMP and autograft groups, no adverse effects such as dysphagia, better pain outcomes for the Infuse group, and an incidence of asymptomatic ectopic bone formation in 11% (two patients) in the BMP group and 6.6% (one patient) in the control group (Fig. 117.2) (18). One collagen sponge soaked in 0.4 mL of 1.5 mg/mL rhBMP-2 was used inside a fibular allograft ring with a final rhBMP-2 dose of 0.6 mg per surgical level. A critical review of this study must include mention of its limited statistical power due to the low patient numbers and the fact that pain at the graft donor site is a well-known comorbidity of iliac graft harvesting procedure. Another early pilot study on the use of poly(L-lactide-co-D,L-lactide) bioabsorbable implants in combination with rhBMP-2 for ventral cervical interbody fusion reported a 5% (one patient) incidence
of severe dysphagia, which required a feeding tube placement until the patient was able to swallow at 1 month postsurgery, although this was the third anterior cervical discectomy and fusion (ACDF) procedure for this patient. Fusion was reported to be successful in 100% of patients. Authors of this study do not report a specific dose for Infuse used, stating only that they followed manufacturer’s instructions (19). However, conclusions from this study are also limited due to the small number of patients, as well as the lack of a control group.
of severe dysphagia, which required a feeding tube placement until the patient was able to swallow at 1 month postsurgery, although this was the third anterior cervical discectomy and fusion (ACDF) procedure for this patient. Fusion was reported to be successful in 100% of patients. Authors of this study do not report a specific dose for Infuse used, stating only that they followed manufacturer’s instructions (19). However, conclusions from this study are also limited due to the small number of patients, as well as the lack of a control group.
Another group reported their results for use of rhBMP-2 placed within a polyetheretherketone cage for ventral cervical fusion. Fusion was reported to be achieved in 100% with 8% (n = 2) of patients developing transient dysphagia and 12.5% (n = 3) developing asymptomatic ectopic bone formation. The initial dose used by the authors of this study was 2.1 mg per surgical level; however, after asymptomatic heterotopic bone formation was noticed, rhBMP-2 dose was reduced in half (1.05 mg per surgical level) for future procedures. No further heterotopic bone formation was observed after BMP dose adjustment, implying the dose dependency of this radiographic finding (20). The authors of this study also reported using a 10-mg dose of dexamethasone at the start of the procedure for all patients undergoing ACDF and a temporary soft tissue drain for multilevel procedures. This study again lacked a control group.
SOFT TISSUE EFFECTS
The early enthusiasm for extension of BMP use to ventral cervical fusion was clouded by several reports of significant complications associated with rhBMP-2 use in such surgeries. Two independent groups reported 23.2% and 27.5% incidence of significant postoperative complications following rhBMP-2 use for ventral cervical fusion (21,22). One of these studies reported outcomes of 151 patients that underwent either an ACDF (n = 138) or vertebrectomy (n = 13)
using Infuse. Among the postoperative complications, hematoma was the most common, occurring in 15 patients (9.9%). Eleven of these patients developed hematoma on postoperative day 4 or 5 and 8 of them required surgical hematoma evacuation. In this study, another 8.6% (13 patients) experienced prolonged hospital stay (>48 hours) or readmission for soft tissue swelling and breathing difficulties without hematoma evacuation or reoperation. As for prior clinical studies, lack of a control group weakens this study. Nonetheless, this study raises the possibility of a link between these complications, the surgery performed, and the dose of BMP used. In our practice, we have also experienced a case of postoperative hematoma after rhBMP-2 use that became symptomatic on the 7th postoperative day with difficulty swallowing and required evacuation via needle aspiration (Fig. 117.3).
using Infuse. Among the postoperative complications, hematoma was the most common, occurring in 15 patients (9.9%). Eleven of these patients developed hematoma on postoperative day 4 or 5 and 8 of them required surgical hematoma evacuation. In this study, another 8.6% (13 patients) experienced prolonged hospital stay (>48 hours) or readmission for soft tissue swelling and breathing difficulties without hematoma evacuation or reoperation. As for prior clinical studies, lack of a control group weakens this study. Nonetheless, this study raises the possibility of a link between these complications, the surgery performed, and the dose of BMP used. In our practice, we have also experienced a case of postoperative hematoma after rhBMP-2 use that became symptomatic on the 7th postoperative day with difficulty swallowing and required evacuation via needle aspiration (Fig. 117.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree