INTRODUCTION
Botulinum neurotoxins (BoNTs) are highly poisonous substances that can be efficaciously used as medications to treat movement disorders. Accidental poisoning leads to botulism, a potentially deadly disease commonly caused by ingestion of contaminated food. The active BoNT (150 kDa) occurs naturally as part of a high-molecular-weight complex containing the neurotoxin moiety and a set of complexing proteins of clostridial origin (1). Complexing proteins, which protect BoNT avoiding destruction in the hostile environment of the gastrointestinal tract, are constituted by hemagglutinin and nontoxin non-hemagglutinin proteins that spontaneously associate with the neurotoxin moiety following their cosynthesis by the bacteria. They help stabilize and protect the neurotoxin moiety from changes in temperature, low pH, and enzymatic degradation (2).
The 150-kDa BoNT is initially inactive; to become activated, it must be nicked (i.e., cleaved by a protease), which produces two polypeptide fragments: a ≈ 100-kDa heavy chain and a 50-kDa light chain that remain tethered together by a disulfide bond. Commercially available type A BoNT (BoNT/A) is approximately 95% nicked (and therefore activated) by an endogenous bacterial protease before it is released from the clostridium. By contrast, type B BoNT (BoNT/B) is endogenously nicked to a much lower level, so this toxin must be exposed to proteases during the manufacturing process, which still leaves approximately 25% to 30% of inactive BoNT/B product in the vial (3). The manufacturing process also includes partial or complete purification of BoNT from complexing proteins.
BoNTs are sequence-specific endopeptidases, which are internalized by nerve endings and inhibit neurotransmitter release by cleaving SNARE proteins (4) (Fig. 46.1). Muscle weakness is produced by action of BoNTs at the neuromuscular junction where release of acetylcholine is inhibited; additional clinical signs observed in botulism depend upon action of BoNTs on autonomic and sensory neurons. There are seven BoNT serotypes, named from A to G; BoNT/A is the most diffuse in medical practice because of its long duration of action (5).
BoNT/A has been introduced in clinical practice in 1980 by Alan Scott following a suggestions by Edward J. Schantz, who provided him with “crystalline” BoNT/A. The lot first used in the clinic to treat ocular misalignment (lot 79-11) was then marketed in the United States of America as Botox®. At about the same time, the UK Centre for Applied Microbiology and Research in Porton Down made available for compassionate clinical use in Europe a BoNT lot developed in that laboratory, which was later marketed as Dysport®. The original Botox® lot was replaced in 1999 by a new one containing less-complexing proteins. BoNT/B was later developed and marketed as Myobloc®/Neurobloc®, and finally, a third BoNT/A product containing virtually no complexing proteins was developed in Germany and marketed as Xeomin®. Other BoNT/A products have recently become available, including Neuronox® (Medytox, South Korea) and CBTX-A (Lanzhou Biological Products Institute, China).
BoNT has proven to be a very flexible tool for the treatment of movement disorders and other medical conditions, with more than 50 proposed indications. With the exception of anal fissure, BoNT does not provide a cure, rather a symptomatic remedy through topical chemodenervation. By reducing the release of neurotransmitters in the injected area, BoNTs reduce the activity of striated or smooth muscles, the secretory function of glands, and possibly the release of sensory transmitters. BoNTs produce a chemical denervation that is topical, reversible, and dose-dependent. BoNT clinics are run by experienced doctors who suitably blend sapient artisan skills with anatomical knowledge.
BOTULINUM NEUROTOXINS
SITES OF ACTION OF BONTS
There are three steps involved in BoNT-mediated paralysis: internalization, disulfide reduction and translocation, and inhibition of neurotransmitter release (see Fig. 46.1). BoNTs bind to the presynaptic nerve ending and enter by receptor-mediated selective and saturable endocytosis.
Following injection into a striated muscle, BoNT produces a chemical denervation of neuromuscular junctions, resulting in weakness and atrophy of the injected muscle. Targeting the endplate motor zone can increase the efficacy of intramuscular BoNT injections (6). BoNT acts both on intrafusal and extrafusal nerve endings. Following BoNT denervation of γ-motor neurons, muscle spindle activity is decreased and the inflow directed to the spinal α-motor neurons is reduced (7). Since muscle activity is supported by afferent feedback, there may be a reduced α-motor neuron drive. Nerve terminals containing active BoNT do not degenerate; neurotransmitter release is blocked by irreversible inactivation of SNARE proteins to restart again when BoNT is degraded by proteolysis, and new synthesis of SNARE protein occurs. Muscle denervation promotes a compensatory sprouting of nerve terminals, leading to the formation of new synaptic contacts. This usually takes from 2 to 3 months and becomes a relevant phenomenon when repeated BoNT injections are performed in the same site.
The limiting factor for the action of BoNTs is receptor-mediated endocytosis, with at least three different groups of presynaptic BoNT receptors, each with different serotype specificity, described at nerve terminals. Their exact distribution on different presynaptic terminals is still not perfectly known (8). In addition to motor neurons, BoNTs are taken up also by autonomic nerve endings, thereby blocking parasympathetic and postganglionic sympathetic cholinergic nerve synapses. This allows using BoNT to treat overactive smooth muscles (e.g., in the case of anal fissure or of esophageal achalasia) and secretory disorders (e.g., focal hyperhidrosis, gustatory sweating, and sialorrhea) (9). Finally, BoNT/A also acts on sensory neurons, inhibiting release of mediators involved in nociception (10).
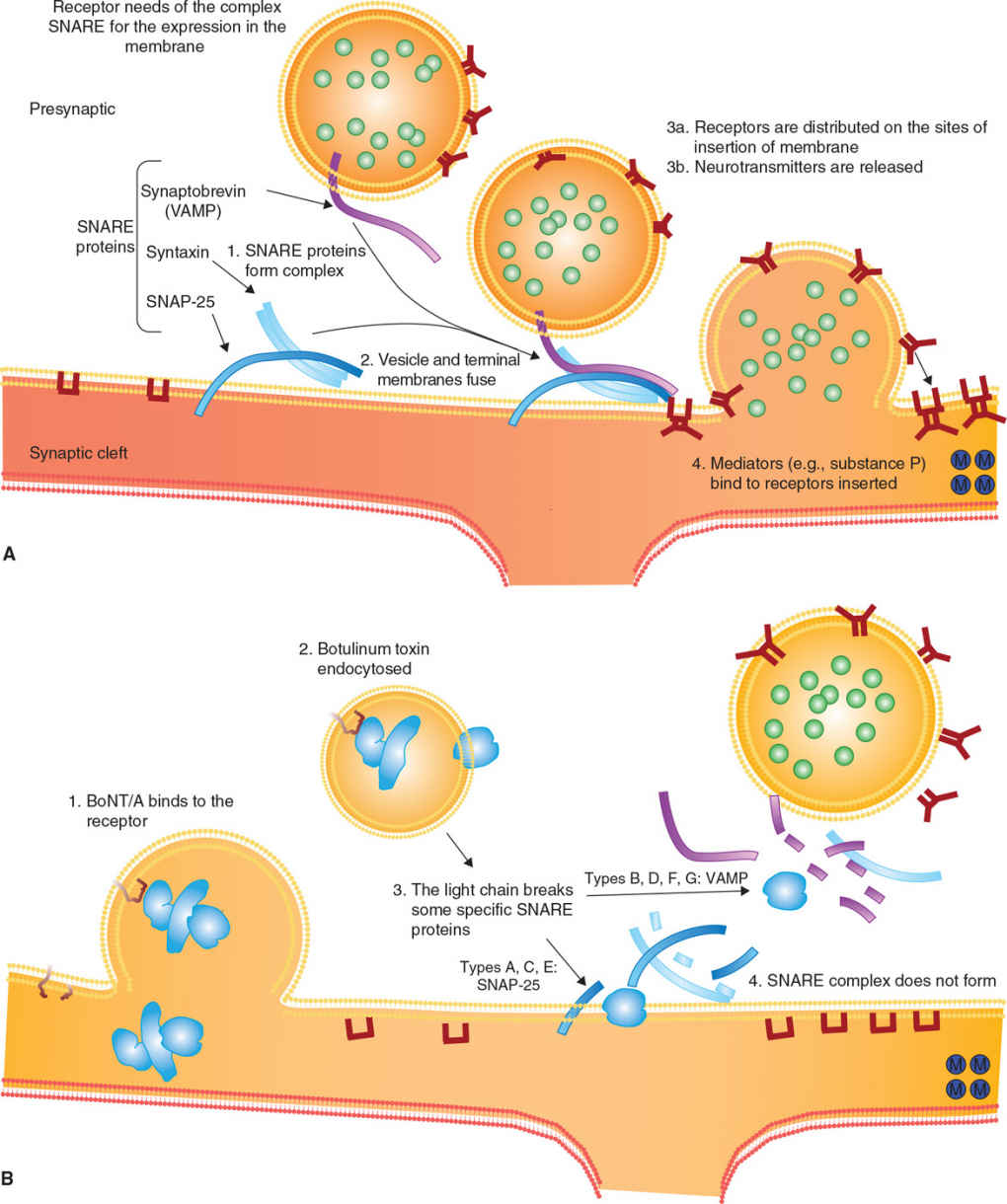
Figure 46.1. Mechanism of action of botulinum toxin. (A) Release of acetylcholine at the neuromuscular junction is mediated by the assembly of a synaptic fusion complex allowing the membrane of the synaptic vesicle containing acetylcholine to fuse with the neuronal cell membrane. The synaptic fusion complex is a set of SNARE proteins. After membrane fusion, acetylcholine is released into the synaptic cleft. (B) BoNT binds to the neuronal cell membrane at the nerve terminus and enters the neuron by endocytosis. The light chain of botulinum toxin cleaves specific sites on the SNARE proteins, preventing complete assembly of the synaptic fusion complex and thereby blocking acetylcholine release. (Modified from Arnon SS, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2001;285:1059–1070.)
COMMERCIALLY AVAILABLE NEUROTOXINS
Four BoNTs are widely available for clinical practice: three are serotype A and one is serotype B. These toxin brands differ for specific activity, packaging, constituents, excipients, and storage. Their main features are summarized in Table 46.1. There are three type A and one type B brands: onabotulinumtoxinA (A/Ona, Botox®, Allergan), abobotulinumtoxinA (A/Abo, Dysport®, Ipsen), and incobotulinumtoxinA (A/Inco, Xeomin®, Merz); rimabotulinumtoxinB (B/Rima, Myobloc®, USWorldMeds; Neurobloc®, Eisai). A/Abo, A/Inco, A/Ona, and B/Rima are all licensed worldwide for cervical dystonia. In addition, the three BoNT/A products are approved for blepharospasm and focal dystonias, spasticity, hemifacial spasm, hyperhidrosis, and facial lines, with remarkable regional differences.
The potency of BoNTs is indicated by using proprietary units derived from mouse protection assays. There is currently no formula to reliably convert proprietary units of one BoNT brand to another, which may cause difficulties when switching between toxin brands in patients already under treatment. A rough estimate is that 1 A/Ona or A/Inco U corresponds to 3 to 5 A/Abo U, and to 40 to 50 B/Rima U. However, the clinical equivalence varies based on the indication, the injected site, and the dilution. There is evidence from preclinical and clinical study that A/Inco U compare to A/Ona U at a 1:1 ratio (11), whereas no data support an unequivocal correspondence between A/Abo and A/Inco. A critical review of the available evidence on the comparability of A/Ona and A/Abo has concluded that there are relevant intrinsic differences not allowing to identify a consistent bioequivalence (12). These two BoNT/A products differ in potency and tendency to diffuse and their equivalence presumably varies according to the body part injected. Furthermore, dilution upon reconstitution, syringe size, and needle gauge all affect the local diffusion of BoNT when injected.
BoNT/A is the first-choice treatment for approved indications, with the most convenient trade-off between efficacy, duration of action, and tolerability. BoNT/B has a shorter duration of action and more side effects than BoNT/A (13,14).
PRIMARY AND SECONDARY FAILURES
BoNTs are usually employed as a treatment for chronic disorders and patients usually require repeated treatments for many years. The term “primary failure” indicates those cases where no clinical efficacy is ever observed after BoNT treatment, whereas “secondary failure” indicates such cases in which initially efficacious BoNT treatments are followed by loss of clinical benefit after repeated treatment sessions. Inappropriate muscle selection or wrong BoNT dosing/targeting is considered to represent the most common causes of secondary nonresponse. In keeping with this, it has been observed that neutralizing antibodies occur only in a minority of secondary nonresponding patients with dystonia (15). Patients may occasionally develop antibodies that may be neutralizing or nonneutralizing. Neutralizing antibodies are primarily directed against the BoNT heavy chain, although neutralizing antibodies that bind to epitopes on all regions of BoNT have also been observed (16). Neutralizing antibodies can inhibit the biologic activity of BoNT, possibly blocking its interaction with the neuronal receptor (17). By contrast, nonneutralizing antibodies are produced either against the nontoxic complexing proteins or bind to BoNT without affecting its biologic activity (18). Clinical data collected before 1999 with the old 79-11 Botox® lot cannot be compared with those obtained with currently available BoNT/A products, which exhibit a low incidence of neutralizing antibodies.
Three clinical tests are commonly used to evaluate the efficacy of BoNT-induced chemodenervation with a high sensitivity rate, by injecting unilaterally any of the following muscles: the frontalis, the eyebrow corrugator, or the extensor digitorum brevis. In the first two cases, asymmetry of forehead wrinkling or glabellar furrowing is evaluated; following injection in the extensor digitorum brevis, instead, the pre- and postinjection changes in action potentials are compared with EMG. Patients who do not respond to one of these clinical tests are likely to have developed neutralizing antibodies against BoNT. The clinical approach is preferred to detection of antibodies by laboratory test, which is difficult to perform and does not reliably predict the outcome to BoNT treatment (19).
MUSCLE SELECTION AND TARGETING
Detailed clinical examination to determine the affected muscles is crucial, as outcome to BoNT treatment is highly dependent on recognition of the muscles primarily involved by dystonia that need to be distinguished from those involved in compensatory activity. Muscle selection is based on inspection, palpation, EMG recording, and ultrasound identification. Inspection allows to detect hypertrophy or atrophy of superficial muscles and to recognize which muscles are involved in postures and movements; palpation is used to identify size and activation of superficial muscles and to evoke pain in contracted muscles. EMG recordings show muscle activity at rest and during voluntary tasks; simultaneous mapping of several muscles is particularly useful for recognizing their dystonic features and their activation/deactivation pattern. Ultrasound allows for a visual recognition of muscles, showing their contractile activity at rest or during voluntary tasks and measuring their size to quantify BoNT-induced atrophy.
Direct injection is normally performed around the eye, where BoNT is actually not targeted to superficial and very thin muscles, rather skillfully placed in the skin. In other territories, there are muscles easily reached by inspection/palpation, such as the sternocleidomastoid, which can be gently clamped with fingers for more than half its perimeter. Other muscles, particularly deep ones, can be injected directly, although they are better targeted using EMG or ultrasound. The latter technique, in particular, allows to directly observing outflow from the needle tip and diffusion from the injection site. Factors, such as dilution and reconstitution, needle gauge, anatomical approach, muscle location, and size, affect the clinical outcome. BoNT can also spread via the lymphatic system or even the bloodstream reaching distant sites, where it may produce subclinical effects (20).
In current clinical practice, simple forms of dystonia are usually injected without guidance, although EMG-guided targeting has proven superior in cervical dystonia (21). For example, in rotational torticollis it is possible to rely on direction, hypertrophy, and pain to choose the combination of muscles to inject. In case of insufficient outcome or when deeper muscles need being injected, EMG- or ultrasound-based targeting is definitely required. Some deep muscles, such as the longus collis, should only be injected with guidance (22,23). As a general rule, complex forms of dystonia require guided inspection and targeting in order to deliver appropriate treatment to the deep muscles involved.

BONT IN MOVEMENT DISORDERS
DYSTONIA
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both (24). This has been the first movement disorder treated with BoNT (25), which stands as the first-line treatment for most isolated dystonia syndromes, particularly those with focal or segmental distribution. Multifocal or generalized forms can be dealt with as a collection of focal treatments. If dystonia is not isolated, such as when combined with parkinsonism, myoclonus, etc., an evaluation of treatment goals should be performed in advance.
Treatment with BoNT is considered the first-choice option for cranial, cervical, and laryngeal focal dystonias (26). Recently, an expert panel evaluated evidence at several levels, supporting BoNT/A or BoNT/B, including each of the commercially available formulations, and made recommendations for therapeutic indications based upon the strength of clinical evidence (27). These recommendations will be reported in the following sections. Overall, BoNTs provide not only safe and effective symptomatic relief but also long-term benefit, favorably modifying the natural course of dystonia (28).
Blepharospasm
Blepharospasm is caused by dystonic contractions of the orbicularis oculi, often accompanied by overflow activation of surrounding muscles, including the procerus and the corrugator. The isolated form is also called “benign essential blepharospasm”; when it is associated with oromandibular dystonia, it is called “Meige syndrome.” The excessive (intermittent or persistent) involuntary closure of the eyelids is usually bilateral, though it may sometimes be unilateral at onset, or evidently asymmetric. Over time, the spasms may become more frequent and continuous, leading to sustained eyelid closure and functional blindness.
The efficacy of BoNTs in blepharospasm has been confirmed by more than 50 open-label studies (that have recruited over 2,500 patients) and by few controlled studies. There are scanty data from controlled trials regarding optimal dosing and no good-quality data for BoNT/B, although it can be assumed that this may be as efficacious as BoNT/A. Common doses are 20 to 40 A/Ona or A/Inco U, 75 to 175 A/Abo U, or 2,500 B/Rima U. The average latency from injection to onset of improvement varies from 3 to 5 days; a benefit lasting for 2 to 3 months has been observed in almost all patients (27). Side effects occur in less than 10% of treated patients (in 8.29% in a large retrospective series) (29). They include ptosis, blurring of vision, diplopia, tearing, and local hematoma, and normally resolve within 2 weeks (30).
Spasms may differentially affect the three concentric parts of the orbicularis oculi muscle, and inadequate results are obtained if the toxin is injected in the orbital portion in patients with a predominant involvement of the pretarsal part (31,32). Furthermore, patients with a predominant pretarsal involvement may have a prevalently tonic eye closure, causing a difficulty in voluntarily opening the eyelids, so-called eyelid-opening apraxia (33). EMG allows recognizing loss of the normal reciprocal inhibition between the levator palpebrae and the pretarsal orbicularis, associated with cocontraction.
Many patients with typical blepharospasm benefit as well from the injection in the orbital or in the pretarsal portions of the orbicularis oculi muscle, although some of them (particularly those with atypical presentations, such as eyelid-opening apraxia) only respond to injections placed in the pretarsal part. A study compared the efficacy of two injection techniques, orbital versus combined (orbital and pretarsal) in patients with unsatisfactory response to BoNT. Nineteen patients with typical blepharospasm not having satisfactory outcome after orbital injections (primary or secondary nonresponders) had a 28% motor improvement and a 47% functional improvement with the combined injection (34). This highlights the importance to refine BoNT targeting in patients with insufficient outcome, allowing a successful outcome in virtually all blepharospasm patients.
Long-term data on BoNT/A have been reported by six studies (35). Efficacy of treatment is considered to remain stable and predictable in the long term following repeated treatment sessions, although the duration of benefit has been reported to vary, increasing or contrastingly decreasing with time. Although it has been suggested that secondary failure to BoNT/A may occasionally develop, long-term data on repeated injections with BoNT/A in blepharospasm did not report any failure (30).
Cervical Dystonia
Cervical dystonia is the most common form of adult-onset focal dystonia, with prevalence estimates of 28 to 183 cases per million and an approximate 2:1 female:male ratio (36). Although muscle involvement almost invariably extends beyond the neck region, cervical dystonia is conventionally considered a focal form. In cervical dystonia, there is a typical combination of dystonic movements and postures pivoting at different points of the cervical spine. Dystonic movements can be fast and irregular in amplitude, from jerky to tremulous. Dystonic postures may occur in a wide range of combinations as the cervical spine has several degrees of freedom and complex muscle arrangements. Recently, a “caput-collis” classification of cervical dystonia postures has been proposed to serve also as a tool to choose injection sites (37). Dystonic malposturing may affect only the cervical spine (collis: 20%) or only the head (caput: 19%) or a combination (61%). The limit of such approach is that abnormal postures are just one component of the complex phenomenology of cervical dystonia. Injection sites are usually chosen by a stepped and flexible approach considering also features different from postures (e.g., dystonic movements, pain, hypertrophy, atrophy). In keeping with this, it has been demonstrated that fixed BoNT doses and rigid muscle selection criteria provide less than optimal results (38).
At variance with other focal dystonias, pain is a hallmark feature of cervical dystonia that occurs in over two-thirds of patients (39). Pain is one of the main reasons for patients to seek treatment for cervical dystonia and is relieved in 90% of patients treated with BoNT/A (40). Interestingly, there is no correlation between the severity of motor signs and duration (or intensity) of reported pain, which occurs regardless of whether muscles sustain or oppose the dystonia or are not even involved in it (41).
There is ample evidence that BoNTs (either A or B type) exert safe and effective symptomatic relief also in the long-term, and favorably modify the natural history of cervical dystonia lowering the risk of contractures and complications for patients (42,43). Table 46.2 provides a listing of cervical muscles commonly injected and indicates average BoNT doses for each muscle. Fig. 46.2 shows typical injection sites for the most commonly targeted muscles. In experienced hands, the adverse events associated with repeated BoNT treatments are minor, self-limiting, and decrease over time (35). The efficacy and tolerability of BoNTs for the treatment of cervical dystonia are supported by Class I evidence (27). In patients refractory to BoNT treatment, deep brain stimulation of the globus pallidus internus may be considered (44). A double-blind controlled study on the efficacy of deep brain stimulation in cervical dystonia is currently underway in Germany.
Seven randomized controlled trials on cervical dystonia, encompassing a total of 233 patients, demonstrated beneficial effects (between 66% and 80% of subjective improvement) of repeated BoNT/A injections (45). The average total dose injected was 100 to 300 A/Ona or A/Inco U, 400 to 800 A/Abo U, or 10,000 to 20,000 B/Rima U. Doses can vary considerably according to individual needs, and this is the focal dystonia where the highest BoNT doses are used. Most studies report that the latency of clinical action is on average around 1 week; the average duration of efficacy is traditionally reported to be around 12 weeks for BoNT/A, based on evidence collected with the original 79-11 lot that is currently no longer used (46). The observation of immunoresistance with that batch has led to identify a minimum 3-month recommended injection interval (47) that is reported in all approved labeling for BoNT products. It has been recently observed that a number of patients would prefer shorter injection intervals than 12 weeks (48), and recent evidence has also been gathered a flexible injection intervals (≥ 6 weeks using A/Inco) provided sustained efficacy and were well tolerated (49). Many studies have demonstrated that the benefits from BoNT can be sustained for many years or decades, and no permanent adverse effects to BoNT have been reported (42).
| Muscles Commonly Affected in Cervical Dystonia, Their Function, and BoNT Doses Currently Used |
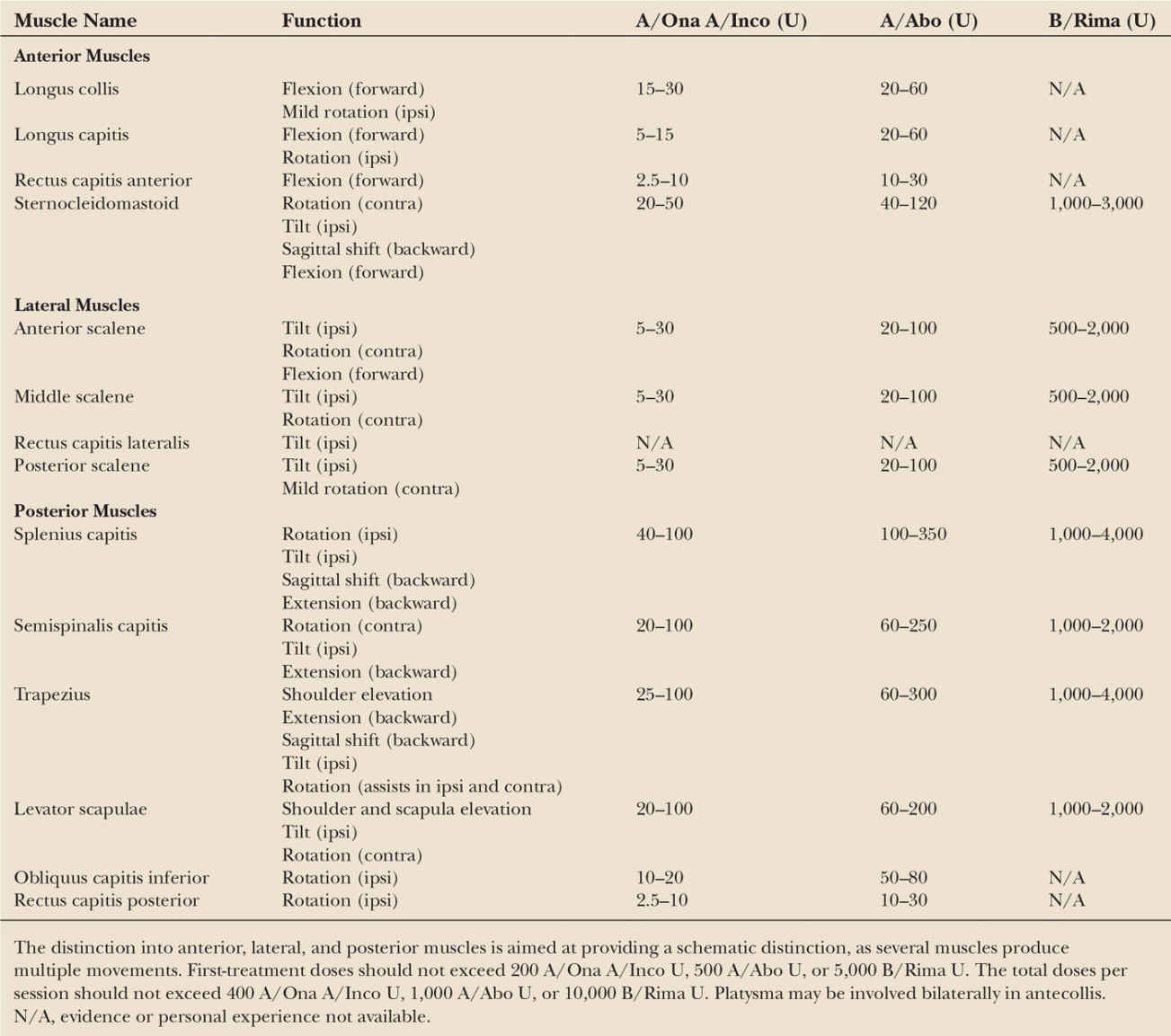
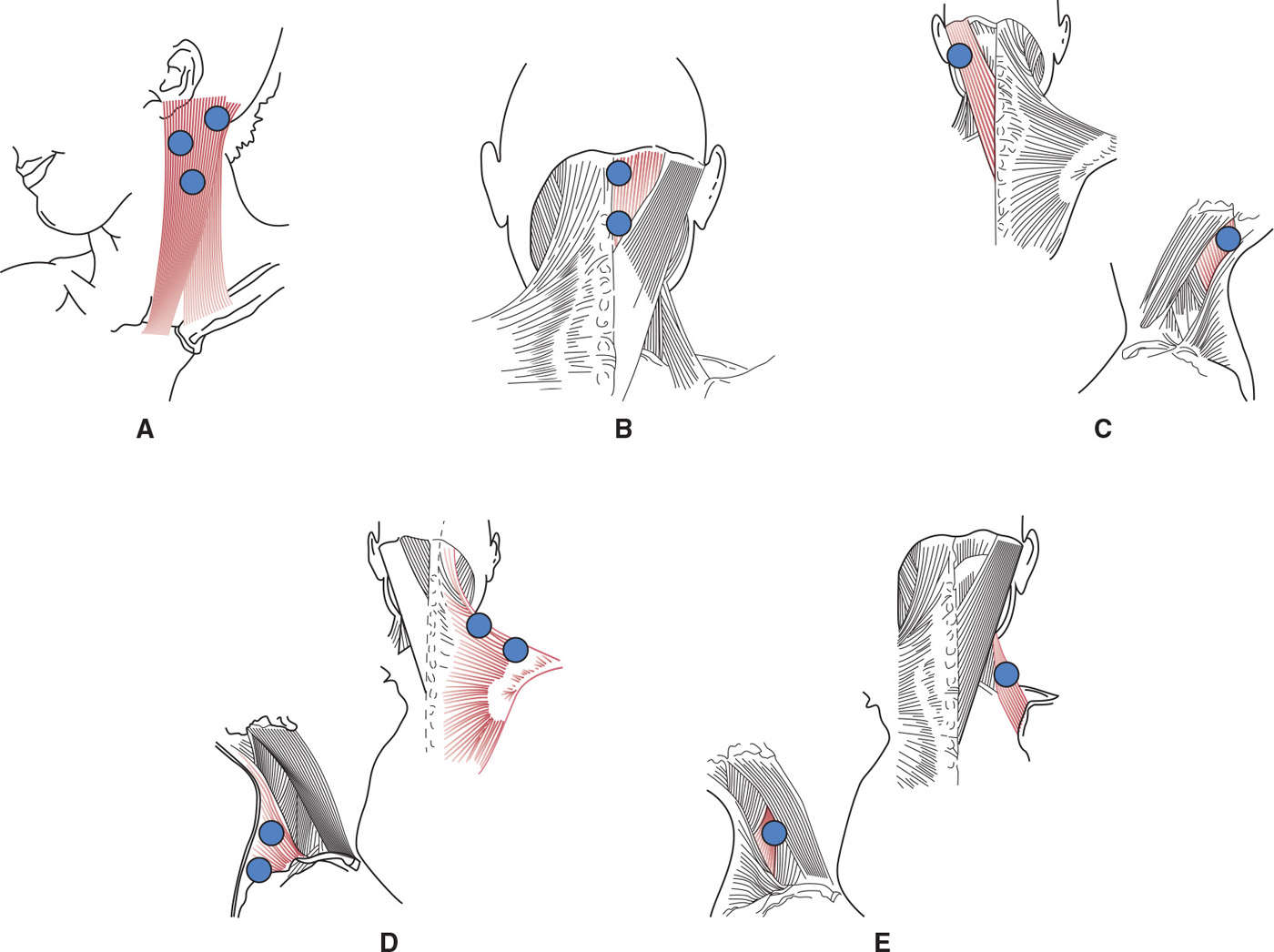
Figure 46.2. Typical injection sites in superficial neck muscles involved in cervical dystonia. (A) Sternocleidomastoid injected into three sites located in the upper part of the muscle; (B) semispinalis capitis injected in two sites to cover its extension; (C) splenius capitis injected in one site; (D) trapezius injected in two sites along its horizontal branch; (E) levator scapulae injected in one site. Average doses are reported in Table 46.2.
The efficacy of BoNT treatment on pain associated to cervical dystonia has been recognized since early experiences (46) and repeatedly confirmed. The results of CD PROBE (Cervical Dystonia Patient Registry for Observation of OnabotulinumtoxinA Efficacy), a prospective, multicenter, observational registry that enrolled subjects with cervical dystonia from January 12, 2009 to August 31, 2012, at 88 sites in the United States, elucidated the occurrence of pain and its impact upon work and treatment patterns. Most subjects reported pain at baseline that correlated with cervical dystonia severity and disability, including work and employment measures (50). Subjects with moderate/severe pain at baseline received a significantly higher mean dose and had a more muscles injected upon initial treatment. Thus, pain must be considered as an important factor when determining the doses and sites injected.
With regard to the motor phenomenology, BoNTs are more efficacious on the postural component than on fast dystonic movements. The most severe side effect and dose-limiting factor is dysphagia, with an incidence of 10% to 40%, depending upon the study, and based on the dose administered and technique used (51). Expert management of injections placed in the sternocleidomastoid and the underlying pharyngeal and laryngeal muscles is required. There is no firm evidence of differences in outcome using either BoNT/A brand, and BoNT/B is considered not to be less efficacious than BoNT/A in cervical dystonia, although duration of benefit is shorter (52). BoNT/B is more likely to produce dry mouth, presumably because it is more potent in blocking cholinergic release in postganglionic parasympathetic fibers to the salivary glands. BoNT/F has also been tested in cervical dystonia; the duration of efficacy, however, is much shorter and lasts for approximately 8 weeks (53).
A recent study attempted to clarify the reasons that lead patients to discontinue treatment (54). Inadequate response was often cited, although a significant minority of patients mentioned addressable barriers in accessing specialty care. Unrealistic expectations may also induce disappointment and, ultimately, treatment discontinuation. Treatment must be individualized and tailored to the specific patient’s needs. Furthermore, BoNT treatment for cervical dystonia should be part of a comprehensive approach, including physical treatment and retraining techniques. Rehabilitation and biofeedback are beneficial addition to BoNT treatment in cervical dystonia, as they improve outcome and may reduce the dose needed for subsequent reinjections and facilitate muscle selection. Physical therapy alone does not provide adequate results in cervical dystonia (55) and should be considered complementary to BoNT. Patients with cervical dystonia are slow in mental rotation of stimuli representing body parts, including hand, foot, and head (56). This abnormality may derive from a defective integration of body- and world-related knowledge, a process that can be improved by biofeedback and visuospatial retraining.
Laryngeal Dystonia
Laryngeal dystonia encompasses several clinical phenotypes characterized by abnormal activity of the muscles of the larynx. The most common subtypes are adductor or abductor spasmodic dysphonia, whereas less common subtypes include laryngeal breathing dystonia (also known as dystonic respiratory stridor) and singer’s dystonia (57). In adductor type spasmodic dysphonia, there is irregular hyperadduction of the vocal folds during speaking, producing a “strained-strangled” voice; in abductor spasmodic dysphonia, there are irregular and inappropriate abductor spasms during speaking, producing breathy breaks or whispering. These two are task-specific dystonias in which laryngeal spasms typically occur during speaking and cause intermittent voice breaks. Laryngeal dystonia must be distinguished from muscle tension dysphonia, in which there is a general constriction of multiple laryngeal muscles leading to a strained voice that does not vary with different parts of speech (58). Many patients with laryngeal dystonia may have features of muscle tension dysphonia that may constitute a behavioral compensation for voice weakness or instability.
Laryngeal dystonia can be treated with BoNT, whereas muscle tension dysphonia can be reversed with voice therapy. Several open studies, overall encompassing over 900 patients, have established the efficacy and safety of BoNT/A in the treatment of adductor and abductor laryngeal dystonia (59). BoNT is considered the treatment of choice despite the fact that no large controlled studies have been performed. Three approaches are currently used in the BoNT treatment of spasmodic dysphonia: (1) unilateral EMG-guided injection, (2) bilateral EMG-guided injection, and (3) injection via indirect laryngoscopy without EMG (60). Most investigators report a 75% to 95% improvement in voice symptoms and a significant improvement in the quality of life, although it has been reported that treatment failures are more common in elderly patients (> 70) (61). BoNT doses used in spasmodic dysphonia can vary depending on the toxin brand and the technique. It has been suggested to start with 0.5 A/Ona U or 1.5 A/Abo U when injecting bilaterally; then, to adjust the dose as needed (the estimated average dose being 0.75–1 A/Ona U or 2–3 A/Abo U) (62). BoNT doses tend to remain stable after the second- or third-treatment session, and so does the treatment interval, leading to a predictable management schedule. Adverse events include transient breathy hypophonia, hoarseness, and occasionally dysphagia with aspiration.
Oromandibular Dystonia
Oromandibular dystonia involves the masticatory, lingual, and pharyngeal muscles. It may have a focal to segmental involvement with jaw-closing, jaw-opening, lateral jaw deviation, or a combination of these abnormal movements. Involuntary tongue and mouth movements, biting of the tongue, cheeks or lips, and difficulty in speaking and chewing produce social embarrassment and functional impairment. Long-term management with BoNT has acceptable morbidity, although the results are less satisfactory than for other focal dystonias. A stepped approach for doses and sites is warranted, and functional outcome is the main goal.
In a large series, 68% of patients had a functional improvement and 31.5% had adverse events, the most common of which were dysarthria and dysphagia (63). BoNT/A injections can be targeted into the masseter and temporalis muscles of patients with jaw-closure dystonia or in the submental muscles and the lateral pterygoid muscles of patients with jaw-opening dystonia. This treatment may markedly improve the symptoms of temporomandibular joint syndrome and other oral and dental problems, as well as dysarthria and chewing difficulties. In a recent uncontrolled series, the following A/Ona doses were injected: external pterygoid (7.5 U), masseter (25–50 U), temporalis (15–25 U), anterior digastric (5 U), platysma (5–10 U), medial pterygoid (20 U) (64). Tongue-protrusion dystonia can benefit from injections into the genioglossus muscle; however, apprehension persists regarding intralingual injections due to the risk of dysphagia (reckoned to affect 14% of treated patients (65)). Still, the advantage is considered to outweigh the risk when patients are treated by experienced injectors.
Upper-limb Dystonia
Upper-limb dystonia often occurs as an occupational cramp while writing, playing an instrument, or performing skilled movements with the upper limb. In such cases, BoNT-induced weakness may impair the fine movements needed to perform skilled professional tasks, a problem particularly encountered with musicians. The number of muscles involved by dystonia may be high, particularly in patients with distal involvement, as there are 23 muscles controlling hand movements and many phenomenologic varieties of hand dystonia. Several small studies have produced conflicting results. In a randomized controlled study, 40 subjects affected by writer’s cramp (the most frequent form of focal hand dystonia) were treated with A/Abo using a flexible scheme, which included variable doses and a retreatment offered after 1 month (66). Muscles were selected for injection according to the pattern of movements and visible or palpable hypertonia. Upon first treatment, finger flexor muscles were injected with 60 A/Abo U per fascicle, finger extensors with 10 to 15 U per fascicle, wrist flexors with 60 to 100 U and wrist extensors with 30 to 40 U. Fourteen patients in the treatment arm (70%), compared to 31.6% in the placebo group, reported a beneficial effect and chose to continue treatment. Side effects were transient hand weakness (reported also in two subjects treated with placebo) and pain at the injection site. In the 1-year follow-up extension, about 50% of patients had remained under treatment with a reported positive effect.
Scanty data are available on the long-term outcome of patients with focal hand dystonia (67). In a recent follow-up of over 10 years, 20 patients with focal hand dystonia were treated with repeated injections of A/Ona (68). It was observed that BoNT remained effective and safe for over 10 years without serious side effects or antibody-mediated resistance. Interinjection intervals were variable, and the dose was increased over time. Musicians had a less-complex dystonia and were more likely to wait longer between injections.
HEMIFACIAL SPASM
Hemifacial spasm is characterized by brief or persistent involuntary contractions of the muscles innervated by the facial nerve that are typically unilateral and may occasionally occur bilaterally. It usually starts in the orbicularis oculi to gradually spread to other ipsilateral muscles, and frequently involves the frontalis muscle and the platysma. The idiopathic form is considered to originate in many cases from a direct contact of the facial nerve with an intracranial artery (e.g., the basilar artery). Microvascular decompression has been proposed as an etiologic treatment for such cases (69). Secondary hemifacial spasm may be caused by a pathologic process developing in the posterior fossa (e.g., neurinoma of the acoustic nerve) or as an aftermath of a previous peripheral facial nerve injury or Bell’s palsy. In such cases, spasms often coexists with mild facial weakness and synkinesias.
Hemifacial spasm impacts a patient’s appearance and persists during sleep, which may cause insomnia. Spontaneous remissions are infrequent and most patients need lifelong symptomatic BoNT treatment or surgical decompression. BoNT/A is the first-choice option, as the available evidence supports a level B recommendation for BoNT/A and A/Ona based on two Class II studies (27). There have been no studies of A/Inco, and there is insufficient evidence to recommend B/Rima. Treatment is usually started by injecting the periocular region, which may be sufficient to improve both the upper and the lower facial nerve territories. Typical starting doses are 10 to 15 A/Ona or A/Inco U and 60 A/Abo U with pretarsal placement. With subsequent treatment sessions, the doses can be increased, and the lower facial component may be targeted. A total dose between 20 and 60 A/Ona or A/Inco U and 40 and 120 A/Abo U is commonly reached. It is not infrequent to reduce the injected doses after some time based on the patient’s response.
BoNT treatments need to be repeated less frequently than for blepharospasm (on average every 10–28 weeks). The duration of efficacy has been reported to increase with repeated treatments, more rarely to decrease or to remain unchanged. It has also been observed that the duration of benefit is shorter in more severe cases (70). BoNT treatment for hemifacial spasm is well tolerated. Focal weakness is the most commonly reported side effect, occurring in 75% to 95% of cases, particularly when injections are placed in the mid or lower face. Ptosis may occur following injections into the orbicularis oculi, with an incidence of up to 53% of cases. In a large series, 3.73% of the patients had adverse events, mainly consisting in ptosis, hemifacial weakness, edema, or bruising (29). Long-term data have shown that either A/Abo or A/Ona is efficacious and safe over 10 years with no major adverse events and a 20% incidence of minor events (71,72).
OTHER MOVEMENT DISORDERS
There is a level B recommendation that BoNT/A can produce modest improvement of (essential) hand tremor (27). A/Ona has been used in all these cases, and no experience has been collected with other formulations. Postural, but not kinetic, hand tremor has been reported to improve (73), with dose-dependent hand weakness as the main side effect.
Tics have been treated with BoNT: the first anecdotal observation was made in patients with dystonic tics (74). Currently, BoNT is used most frequently for eye blinking and neck or shoulder tics. A controlled study showed efficacy of BoNT/A for simple motor tics with a 39% reduction in their frequency and a reduction of premonitory urge (75). Remarkably, however, the patients did not report a comparable subjective benefit. It has also been observed that BoNT treatment reduces in severity premonitory sensations after repeated injections. A study of BoNT on vocal tics also yielded symptomatic and quality of life improvement (76). The overall evidence is however insufficient to warrant recommendations for this indication (27).
Palatal myoclonus has also been treated with BoNT. The experience is limited to few centers. In one recent study, 2.5 A/Ona U was placed into the tensor veli palatini muscle to treat tinnitus or medially in the soft palate, on either side of the uvula, to treat palatal movements (77).
Anecdotal reports indicate that bruxism can also be treated with BoNT injections. Treatment reduces the frequency of bruxism events, decrease bruxism-induced pain, and provides subjective benefit to the patients (78). The doses used vary between 25 and 100 A/Ona U in the masseter and temporalis muscles bilaterally.
SPASTICITY
Upper Motor Neuron Syndrome
Upper motor neuron (UMN) dysfunction can derive from inherited or acquired conditions and is characterized by a combination of spasticity and paralysis leading to a focal or generalized syndrome. In addition to increased tone, other common features of UMN syndrome are increased muscle stretch reflexes, muscle spasms and clonus, weakness (spastic paralysis), and impairment of voluntary movements.
BoNT treatment is primarily aimed at treating spasticity associated with the UMN syndrome, and significant experience has been collected in the acquired condition secondary to spinal cord or traumatic brain injury, multiple sclerosis, or stroke. The primary objectives of spasticity management are to reduce muscle overactivity, and prevent irreversible soft-tissue changes and tendon contractures by maintaining muscle length and normalizing limb positioning. The therapeutic approach to spasticity requires a comprehensive and multidisciplinary judgment of functional goals that exceed the mere reduction of spasticity (79). The upper and lower limbs undergo opposite tone and postural changes following UMN lesion: the upper limbs acquire a flexed posture and mainly loses dexterity, whereas the lower limbs become extended and retain a certain degree of support. Some degree of lower-limb spasticity is a useful support for standing and walking. Therefore, spasticity in the upper and lower limb is addressed as a distinct clinical issue. Tables 46.3 and 46.4 list upper- and lower-limb muscles with average BoNT doses for each of them.
Upper-limb spasticity in UMN syndrome has been the object of several evaluations providing level A recommendation for A/Abo and A/Ona, level B recommendation for A/Inco, and insufficient evidence for B/Rima (80). A large recent trial has suggested that BoNT/A is unlikely to provide sufficient improvement of active upper-limb function in patients with poststroke spasticity, but it may improve basic upper-limb tasks, such as hand hygiene, facilitation of dressing, and pain (81). Fewer publications are available for lower-limb spasticity, which nevertheless provide sufficient clinical evidence to support a level A recommendation for A/Ona and for BoNT/A; the clinical evidence for A/Abo is supported by a level C recommendation, and there is insufficient information to recommend A/Inco and B/Rima (80).
The muscle groups targeted and the doses injected depend on a multidisciplinary assessment focused on functional goals that differ for the upper and the lower limbs. Typical functional goals for the upper limb are reduction in spasticity with increase of range of motion and maximization of residual muscle strength, improvement of care, hygiene, and spatial control. For the lower limb, typical goals include reduction of abnormal posturing, increase of range of motion, and improvement of gait that can be evaluated with instrumental gait analysis. As a rule, the doses of BoNT used in spasticity are higher than those used to treat other movement disorders.
| Muscles Commonly Treated in the Upper Limb, Their Principal Function, and BoNT Doses Commonly Used |