Large/or giant saccular aneurysms ≥ 14 mm
Calcified or atherosclerotic sac, heavily thrombosed
Complex or absent neck
Neck diameter >4 mm
Neck encompasses > 90° of vessel circumference
Dome/neck ratio < 1.5
Atherosclerosis, calcification
Fusiform aneurysms
Blister aneurysms; ruptured aneurysms <2.5 mm
Intracranial dissecting aneurysms (ICA, MCA, VA, BA, PICA)
Branch origin from aneurysm
Origin from sac (not neck)
More than one branch from neck
Bifurcation aneurysm with branches at >180°
Recurrence after prior treatment (clipping, coiling, stent)
Fig. 40.1
Treatment protocol for complex aneurysms
40.2 Parent Vessel Occlusion
When the intracranial internal carotid artery (ICA) is occluded, whether or not a bypass is needed in all patients is controversial. A selective approach is used to base this decision on a balloon occlusion test, with monitoring of cerebral blood flow (CBF) by single-photon emission computed tomography (SPECT), transcranial Doppler ultrasound (TCD), or xenon blood flow studies. A universal approach recommends a bypass in all patients. The senior author followed a selective approach in his earlier patients. However, based on a review of patients who were not revascularized and suffered strokes and the reports of other surgeons with a similar experience [2, 4, 10, 18, 21, 23], a universal approach is presently followed, if the ICA has to be occluded surgically. A selective approach is used in patients who can have a vascular occlusion via an endovascular technique. However, even in such patients, we have observed the occurrence of delayed ischemia, necessitating a protracted, hypertensive management in the intensive care unit or the need for bypass in a delayed fashion in some patients. The potential long-term consequences of large vessel occlusion – increased incidence of flow aneurysms, enlargement of existing aneurysms, and increased incidence of hypertension – have to be kept in mind [5, 24]. Therefore, an endovascular occlusion of the parent vessel harboring an aneurysm is performed presently in patients older than 60 years of age if there is a good collateral circulation demonstrated by anatomic and physiological tests. In the case of the carotid artery, a balloon occlusion test is performed for 15 min under normotensive conditions. Simultaneously, TCD monitoring is performed, making sure that the middle cerebral artery (MCA) velocity does not fall below 30 % of the baseline. Xenon blood flow or SPECT scanning may also be performed when possible to assess the cerebral perfusion. It is important to note the sources of collateral circulation and to ensure that they will not be occluded by the treatment. If all criteria are fulfilled, then the ICA may be occluded with coils. In all other patients wherein the ICA is significantly involved by the aneurysm, a cross-compression angiogram (with compression of the cervical common carotid artery) is performed, to determine the adequacy of the collateral circulation during temporary occlusion at surgery. This enables surgical decision-making regarding the risk of temporary occlusion and selection of the proximal donor vessel for the bypass (e.g., the ICA or external carotid artery, ECA).
More recently, however, endovascular occlusion of the ICA has been replaced by a flow diversion stent (PED) (ev3 Endovascular, Inc., Plymouth, MN, USA) which allows the preservation of the ICA.
40.3 Endovascular Options for Preserving or Reconstructing the Parent Vessel
Balloon-assisted coiling is used for wide-necked aneurysms with a dome to neck ratio between 1.5 and 2. However, there are no long-term studies (>2 years follow-up) demonstrating the durability of aneurysms coiled with balloon assistance. The recurrence rate of wide-necked aneurysms due to coil compaction at short-term follow-up has been 35–60 % [7]. Stent-assisted coiling is an option for more complex aneurysms. It is preferred for older patients because of questions about the durability and long-term safety of endovascular stents. Recurrence rates after stent-assisted coiling have been reported to be around 15 % [11]. When the vessel wall is totally diseased (e.g., dissection, fusiform aneurysms) or when branches arise from the wall, stent-assisted coiling may not be an option. Stent-assisted coiling is also not preferred in a patient with subarachnoid hemorrhage (SAH), since the patient has to be loaded and maintained on antiplatelet therapy with aspirin and clopidogrel. For the same reasons, stent-assisted coiling may be contraindicated even in patients with unruptured aneurysms with associated gastric or duodenal ulceration, other intestinal pathology, or intolerance to antiplatelet therapy.
40.3.1 Flow Diversion Stents (PED)
The PED is a flow diverting device that works on the principle of creating a “new artery.” These are low-porosity stents, with more metal than high-porosity stents, which are used just to prevent coils from occluding the artery [8, 22]. Its on-label use is indicated in intracavernous, ophthalmic, and paraclinoid unruptured aneurysms ≥1 cm. PED requires prolonged dual antiplatelet therapy with monitoring of platelet activity, which demands patient reliability to stick to the medication. On-label use of pipeline device has shown promising results with lower overall morbidity (up to 5 %) and higher aneurysm occlusion rate of up to 90–94 %. The complication data of the on-label use of PED reported in literature showed major stroke (3.2–20 %), minor stroke (1–11 %), intraparenchymal hemorrhage (3.2–11 %), PED thrombosis (4 %), aneurysm rupture (8 %), optic nerve injury (2.7 %), and ICA dissection (0.7 %), and the reported incidence of deaths is up to 6 %. However, the complication rate is much higher with the off-label use of the device [8].
40.4 Microsurgical Options for Reconstructing the Parent Vessel
Clip reconstruction is possible for aneurysms provided that a distinct portion of the artery wall (at least 270° in circumference) is healthy, and not aneurysmal. Potential problems include the need for 15–30 min of temporary occlusion of the artery, multiple trials of clipping, the need for intraoperative angiography, and the occurrence of neck remnant or occlusion of the artery by the clipping process. Frequently, such aneurysms have an atherosclerotic wall or extensive thrombus inside, which complicates the operation. Surgical reconstruction options for complex aneurysms include local techniques such as resuture interposition graft and side-to-side anastomosis, as well as extra- to intracranial bypass techniques using the superficial temporal artery (STA), saphenous vein, or the radial artery. In addition, in some patients, a temporary bypass using the radial artery may be required to provide adequate collateral circulation to the brain during protracted temporary clipping and occlusion of an intracranial aneurysm [12, 16, 17, 19].
40.5 Preoperative Preparation, Anesthesia, and Monitoring
If a bypass procedure is planned to treat an aneurysm, the patient is placed on aspirin, 325 mg by mouth preoperatively for 1 week before the surgery or at least for 2 days prior to the surgery. A prophylactic antibiotic, usually ceftriaxone 1 g, is administered intravenously 1 h prior to the incision and continued for 48 h postoperatively. The patient should have central venous and arterial line monitoring. In case of radial artery grafts (RAGs), the graft extraction site is marked before the operation to avoid the placement of the radial artery line in that arm. When RAG or saphenous vein graft is planned, the vessel is imaged preoperatively by duplex ultrasound and Doppler Allen test (for RAG) in order to evaluate the vessel size and, in the case of the saphenous vein, to mark its course on the skin. A lumbar drain or a ventriculostomy is inserted prior to surgery in order to achieve adequate brain relaxation, in addition to the administration of intravenous mannitol upon skin incision. Total intravenous anesthetic technique (TIVA) is used. Intraoperative neurophysiological monitoring is performed with the monitoring of somatosensory-evoked potentials, electroencephalogram, and motor-evoked potentials. When prolonged temporary occlusion of the intracranial vessels is anticipated during the aneurysm treatment (>30 min), or during the bypass procedure itself, 3,000 units of heparin is given intravenously. Patients are placed in burst suppression using propofol in order to protect the brain during temporary arterial occlusion for bypass [10], but care must be taken to avoid hypotension during such burst suppression. Normal blood pressure and volume are maintained during burst suppression in cases of ruptured aneurysms, and in case of unruptured aneurysm, the blood pressure is elevated 20 % above the patient’s normal value. Autoregulation of cerebral blood flow may be impaired or absent in aneurysm patients with high-flow bypasses used for revascularization. Hence, systemic hypertension (more than 20 mmHg above the baseline) must be avoided after the operation, for 48 h. Postoperative ICG angiography is always performed during the management of complex aneurysms [14, 16, 17].
40.6 Choice of Graft
The choice of graft depends upon four factors: (1) the size of the recipient vessel, which is the major determinant, (2) the availability of a donor vessel, (3) the availability of graft material, and (4) the extent of blood flow augmentation required. A low-flow vessel (<50 ml/min) such as the STA is generally inadequate if a large parent artery, such as the ICA, has to be sacrificed during the bypass operation, unless another collateral source exists (e.g., a good anterior communicating, ACOM, artery). If the STA is large in caliber, it may be anastomosed to an M3 branch of the MCA, and this may provide adequate flow when the volume demand for replacement is low. High-flow grafts (>100 ml/min) such as the RAG or the saphenous vein graft are more reliable when the occlusion of the ICA is planned. The radial artery provides a flow rate of between 100 and 200 ml/min acutely, and the flow can increase significantly over the ensuing days, as measured by TCD [22]. The radial artery is easier to harvest. However, one major problem with its use is the occurrence of vasospasm, which can be prevented by the use of the pressure distension technique [16, 20]. A saphenous vein graft is used when the radial artery is not available as a suitable vessel. It is better to extract the saphenous vein from the upper leg and the lower thigh, where it has a fairly uniform caliber. The saphenous vein has a much thicker wall than the intracranial vessels, and because of the high flow through it, it is more prone to kinking the distal anastomotic site and is technically more difficult to perform than the RAG. Graft flow in the saphenous vein has been measured at 100–350 ml/min. In children below the age of 12 years, where the RAG is small in diameter, the saphenous vein graft is the only alternative. Because of the high flow through the saphenous vein graft, there may be a flow mismatch when it is anastomosed into the MCA; this could lead to turbulence and graft flow problems. Long-term results for saphenous vein grafts and RAGs in the intracranial circulation are not available at present. The long-term patency of both RAG and SVG in the intracranial circulation is good [14].
40.7 Exposure of the STA
The flap technique is used to expose the superficial temporal artery (STA). The superficial temporal artery is mapped out on the skin by means of a Doppler probe. Attention is paid to avoid the artery while opening the skin flap. The artery is dissected from inside after the skin flap is elevated. The skin and subcutaneous tissue flaps are elevated along with the pericranium from the temporal fascia. The STA is dissected from the preauricular region where it lies between the periosteum and the galea, through the point it enters the galea almost to the edge of the skin flap. Some periadventitial tissues are left in place until the end of the artery. It is denuded of branches and left in situ more proximally. Periadventitial tissue is removed near the end of the artery. It is left in situ and is covered with some papaverine-soaked cottonoids until ready to be used for the bypass.
40.8 Radial Artery Exposure
The presence of adequate perfusion to the hand must be confirmed by the Allen test preoperatively, prior to the extraction of the radial artery [3]. During the procedure, a pulse oximeter is placed on the index or middle finger to further test the adequacy of the collateral circulation. The radial artery pulse is palpated just above the wrist and a vertical incision is made through the skin and subcutaneous tissue and deep fascia. This is extended as a gently curved, longitudinal incision on the ventral aspect of the forearm following the course of the artery. The artery is identified distally on the volar aspect of the forearm between the tendons of the flexor carpi radialis and brachioradialis muscles (Fig. 40.2). It is then traced proximally from this point, upward to just below the bend of the elbow, where it lies under the brachioradialis. Once the artery is well exposed, it should be gently occluded between the fingers of the surgeon to verify that there are no changes in pulse oximetry, to confirm the patency of the ulnar artery and the palmar arch. Small arteries and veins emerging from the neurovascular bundle are occluded with tiny titanium clips and further cauterized with bipolar cautery beyond the clip and then divided. The venae comitantes are left attached to the artery except near the ends. The artery is left in situ and harvested just prior to the anastomosis. At extraction, the artery is ligated proximally and distally, sectioned sharply, and removed. It is first cleared of intraluminal blood with heparinized saline using a small blunt needle. Following this, the pressure distension technique (Fig. 40.3) is used to expand the vessel. This is done by compressing the graft around the needle, then pinching the graft sequentially in segments, and distending the graft with heparin saline to expand it. After removing the RAG, the adventitia is removed from the artery at either end of the graft, for about 1 cm.
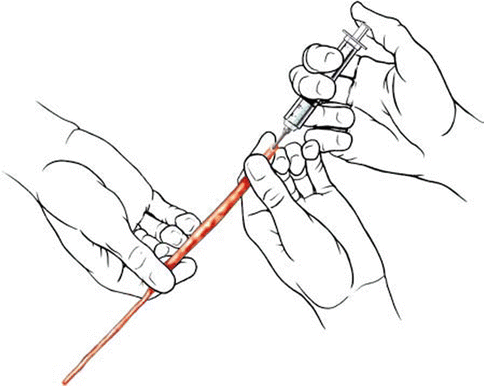

Fig. 40.2
Course of the radial artery in the forearm (Courtesy of Laligam N. Sekhar)

Fig. 40.3
Pressure distention technique (Courtesy of Laligam N. Sekhar)
40.9 Saphenous Vein Exposure
The best way to find the vein is by preoperative duplex ultrasound imaging and mapping. The ideal location for vein harvest is the upper leg and the lower thigh, where the vein is of uniform caliber. During surgery, the leg is positioned with the thigh rotated outward (Fig. 40.4). The vein exposure is started at the lower end and traced up at least 20 cm superiorly; branches are ligated or occluded with titanium hemoclips and divided. The vein is left in situ until extraction. At extraction, both the ends beyond the graft segment are ligated, and the vein is sectioned and removed. After extraction, it is flushed through with heparinized saline and distended at a slight pressure to release the spasm of the vessel. The adventitia of either end of the vein must be trimmed adequately before implantation. The vein should be transferred cranially without reversal, to avoid valve-related blood flow obstruction. The flow direction in the vein is confirmed before anastomosis by flushing the graft with heparinized saline and confirming free flow of fluid from the other end. The vein is marked longitudinally with methylene blue to detect torsion.

Fig. 40.4
Course of great saphenous vein until its drainage into the femoral vein (Courtesy of Laligam N. Sekhar)
40.10 Choice of Recipient Vessel
In order to perform the bypass, the appropriate vessel must be selected beforehand. In the anterior circulation, this is generally an M2 branch of the MCA. The M1 segment is unsuitable for bypass due to the presence of lenticulostriate perforators, and temporary ischemia is poorly tolerated in this vascular territory. The vessel chosen as the recipient should be at least 2 mm in diameter. Saphenous vein grafts require a larger vessel for implantation and it is often best to implant the vein into the bifurcation of the MCA or into a vessel at least 2.5 mm in diameter. In some patients, the MCA branches very proximally with a short M1 segment and the perforators will arise from the various branches. This may make the MCA unsuitable as a recipient vessel for a high-flow bypass. In such cases and if the aneurysm is infraclinoid, then the supraclinoid ICA may be chosen to function as the recipient vessel for the bypass. When the ICA is used in this fashion, the patient must have some collateral circulation from the contralateral anterior cerebral artery, since it may be necessary to temporarily occlude the posterior communicating artery during the bypass. RAGs can be implanted into arteries about 2 mm in diameter.
40.11 Intracranial Anastomosis
We prefer the addition of a skull-base approach to the standard craniotomy to enhance the exposure and to reduce brain retraction in the case of large or giant aneurysms of the ICA [18, 21]. Usually, orbital osteotomy, orbitozygomatic osteotomy, or biorbital osteotomy is performed. Such an approach may not be necessary for distal MCA aneurysms. Prior to dural opening, epidural hemostasis must be carefully secured. This is very important in cases where a bypass is performed because heparin is administered intravenously during the procedure. The dura mater is opened with the aid of a surgical microscope. The early steps of the operation are to expose the aneurysm with minimal trauma to the brain and to occlude very few veins. In cases of paraclinoid and ophthalmic aneurysms, an intradural optic nerve decompression and clinoidectomy are performed. The intracranial anastomosis is performed first to allow the graft vessel to be flipped easily after the first side has been sutured. Generally, the anastomotic time should be less than 45 min and preferably less than 30 min. After the patient has been placed in burst suppression, with the systemic blood pressure being maintained at normal or elevated levels, temporary clips are placed on the recipient artery. An oval-shaped arteriotomy is then created into the recipient artery (Fig. 40.5). In the case of RAGs, this arteriotomy should be about 3–4 mm in length; in the case of saphenous vein grafts, it should be 4–5 mm in length. The distal end of the graft is then anastomosed to the side of the recipient vessel by anchoring sutures on either end of the anastomosis. Following this, the suturing is started on the more difficult side, near the heel of the graft, because this is where leaks are more likely, and a continuous suture is performed. Once the suturing has been performed on one side, the graft is flipped over and the lumen of the vessel is carefully inspected to make sure that there has been no suturing of the opposite wall. Either two running sutures or figure of eight interrupted sutures can be used to complete the anastomosis. Prior to completing the last sutures, the graft is flushed with heparinized saline and a temporary clip is placed on the graft, usually about 2 cm proximal to the distal anastomosis in case of the radial artery and about 1 cm proximal to the anastomosis in case of the saphenous vein (distal to any valves that may exist). The graft is then carried to the donor vessel in the neck area. In case of RAG, the graft may be passed preauricularly or postauricularly, based on its length. Saphenous vein grafts are usually placed postauricularly, avoiding the potential for constriction in the tunnel. With postauricular placement, the graft may be placed under direct vision, and a groove is cut in the bone with the SONOPET ultrasonic bone curette. In the case of the STA-to-MCA anastomosis, the STA is connected to the largest branch of the MCA possible, usually an M3 branch, using 10/0 nylon or 9/0 nylon sutures. A continuous suturing technique is frequently employed [10, 14].
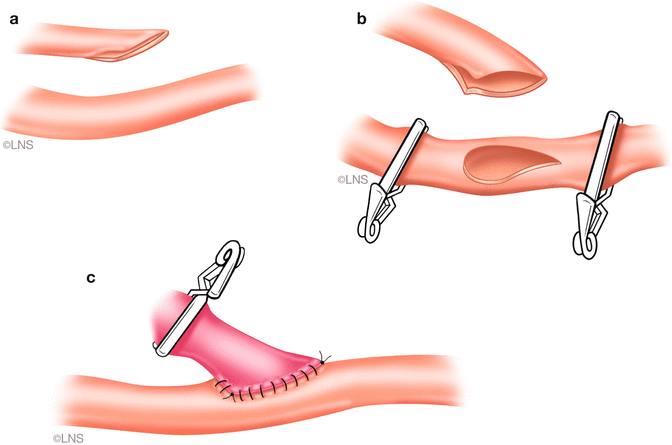

Fig. 40.5
Radial artery graft distal anastomosis. (a) A fish-mouthed graft. (b) Tear-drop-shaped arteriotomy in the recipient vessel. (c) The completed anastomosis (Courtesy of Laligam N. Sekhar)
40.12 Extracranial Anastomosis
We generally perform a high carotid exposure (Fig. 40.6) in order to reduce the length of the graft. If the anastomosis is planned to the ICA (Fig. 40.7), the ICA must be traced above the level of the digastric muscle, which is divided in order to facilitate the exposure. If grafting is into the ECA (Fig. 40.8), it should be exposed for at least 2 cm distal to the carotid bifurcation, until its major branching into the superficial temporal and internal maxillary arteries. The proximal anastomosis is performed either in an end-to-side fashion. A vascular punch is used to create an oval opening, 3.5–5 mm in diameter, and the graft vessel is fish-mouthed to enlarge the opening. In such a case, the graft flow is checked with Doppler measurements and intraoperative angiography after temporary ICA occlusion, and the ICA is permanently occluded if the graft flow is good and fast. Before tying the last suture of the proximal anastomosis, the graft and the recipient vessels are bled by releasing the temporary clips transiently, to clear air bubbles in the case of an RAG; in the case of a vein graft, it is not possible to back-bleed the graft after removing the proximal clips. However, if any air bubbles are observed, these can be extracted using a fine needle placed into the vein just prior to the distal clip on the graft or by opening a side branch to allow blood to wash the air bubbles through. The distal clip on the graft is then removed and the graft is carefully observed and palpated. A micro-Doppler probe is then used to check the graft flow. If a non-quantitative Doppler flow probe (Mizuho micro Doppler, Mizuho America, Beverly, MA, USA) is used, then both systolic and diastolic flow must be present. If a quantitative Doppler flow is employed (Transonic Doppler Flow Probe, Transonic Systems, Ithaca, NY, USA), then one should observe at least 35 cm3 of graft flow. The quantitative Doppler flow probe does not give very accurate measurements of flow velocity in our experience; however, it does give a very clear idea about whether or not flow is present. An ICG angiogram is performed to check Doppler flow and usually replaces an intra-arterial DSA.
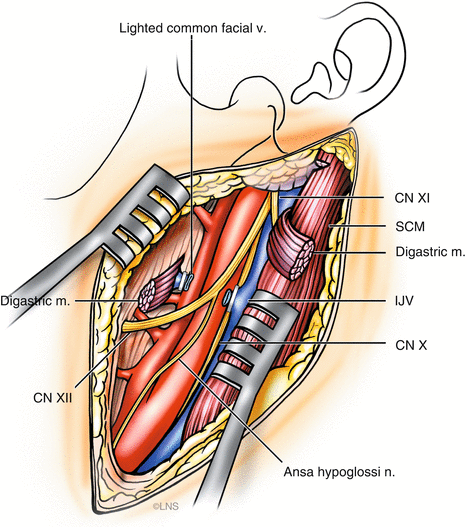
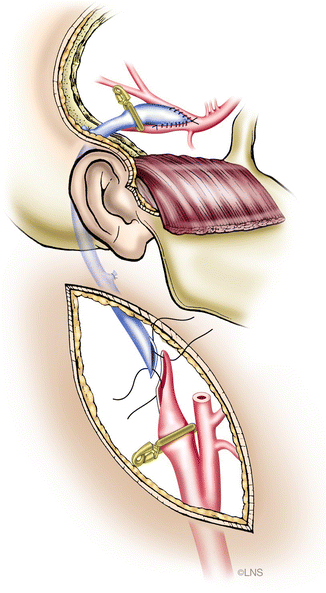
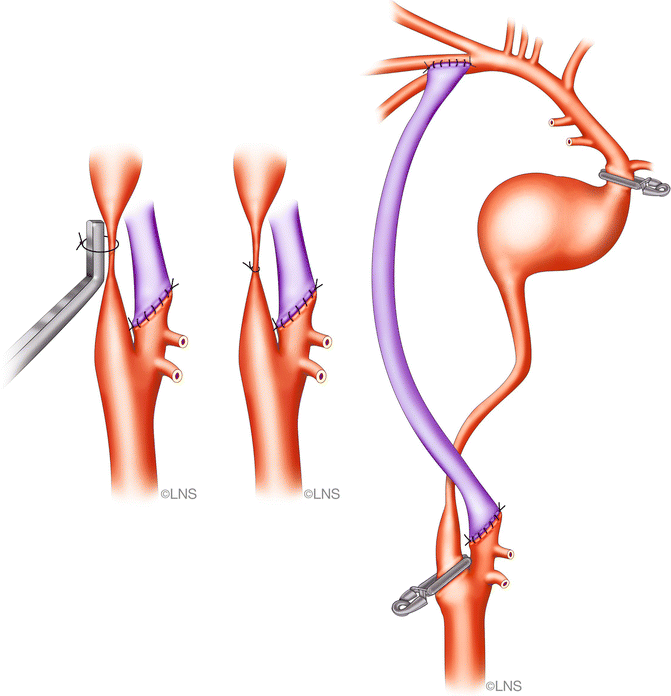

Fig. 40.6
High cervical exposure of carotid arteries for proximal anastomosis (Courtesy of Laligam N. Sekhar)

Fig. 40.7
Cervical internal carotid artery (ICA)-to-middle cerebral artery (MCA) saphenous vein bypass (Courtesy of Laligam N. Sekhar)

Fig. 40.8
Cervical external carotid artery (ECA)-to-MCA bypass (Courtesy of Laligam N. Sekhar)
40.13 Management of Aneurysms
In aneurysms that are extradural (infraclinoid aneurysms), proximal occlusion is adequate. However, optic nerve decompression may be needed, if it is compressed by the aneurysm. In case of intradural aneurysms, it is better to trap the aneurysm. Alternatively, the aneurysm may be clipped with the preservation of perforator vessels, such as the anterior choroidal artery, even though the parent vessel may be occluded in the process. Once the graft has been completed, and is flowing, then one can expect an increase in the intra-aneurysmal pressure, and therefore, the treatment of the aneurysm must be performed fairly quickly. The aneurysm should not be left open for later, because the aneurysm may rupture or the graft may occlude. After the bypass grafting, heparin is not reversed. We always close the neck first, and check the flow in the cranial wound, to make sure that there are no problems with the proximal anastomosis or in the tunnel. Sometimes the graft length may need to be adjusted, in order to eliminate some kinks. The dura mater is closed loosely with sutures and supplemented with dural repair (Confluent Surgical, Waltham, MA, USA). The graft is checked again with TCD after the affixation of the bone flap. A sizeable segment is cut into the bone flap to allow the passage of the graft intracranially and a grove is cut in the bone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








