Fig. 41.1
Management protocol for posterior circulation aneurysms (Note that age is given in years)
41.3 Collateral Circulation and Parent Vessel Sacrifice
The effect of occlusion of the posterior circulation arteries depends upon the collateral circulation. Such collaterals may exist through the circle of Willis (posterior communicating arteries), pial collateral circulation around the cerebellum or brain stem, and deep collaterals through the cerebellum and brain stem. The presence of pial collaterals and perforating collaterals may or may not be observed angiographically and are more likely to exist in young patients without vascular risk factors such as hypertension and absent in older patients with multiple risk factors. When parent vessel occlusion is done, it is best done using an endovascular technique. Major vessel sacrifice may be preceded by a balloon occlusion test for 15 min under normotensive conditions. The actual occlusion is done under general anesthesia, and the patient must be heparinized for at least 24 h perioperatively. The nondominant vertebral artery can be occluded safely provided the posterior inferior cerebellar artery (PICA) is preserved. The dominant vertebral artery (VA) can be occluded if there is one other (small) VA and at least one large posterior communicating artery. With regard to the mid- and lower basilar artery (BA; including vertebrobasilar aneurysms), some type of bypass procedure is necessary unless both posterior communicating arteries are of large caliber >1 mm [7]. The origin of the anterior inferior cerebellar artery (AICA) is an important consideration. In two patients, we performed only a distal occlusion procedure, which successfully preserved the AICA. This is feasible only when the aneurysm is unruptured. In the case of ruptured aneurysms, the aneurysm and the BA can be occluded in a way that preserves the AICA and any major perforators. There is concern about the perforating vessels arising from the mid- and lower basilar segments; in patients with a partially thrombosed aneurysm, these vessels may already be thrombosed, and collaterals are likely to have developed through the brain stem. The length of the artery involved by the aneurysm is important, since the number of perforators in the segment increases with the length of the artery involved. However, the maximum length that can be safely occluded is not known. For complex and giant aneurysms of the upper BA, we have relied on the experience of Drake and Peerless [2, 19] that BA occlusion is safe when both posterior communicating arteries are more than 1 mm in diameter. Many giant aneurysms in this area are very complex and may involve the origin of both PCAs and one PCA. A bypass into the PCA can augment flow and make it safe to occlude the upper BA when the posterior communicating arteries are small in size; the use of the deep hypothermic circulatory arrest technique for clipping of the aneurysm (with neck remnant), the Neuroform stent with endovascular coiling, and bypass into one PCA with aneurysm clipping, or coiling, are treatment options [1, 4, 5, 12, 14]. With respect to second-order arteries such as the PCA (beyond the posterior communicating artery), the superior cerebellar artery (SCA), the AICA, or the PICA, occlusion testing is not possible. In most patients, perforators to the brain stem arise close to their origin from the parent artery, and therefore, occlusion of the proximal segment should be avoided. Effects of occluding the more distal segments are variable and depend on the size of the artery and the collateral circulation available. Occlusion of the P3 segment in the treatment of aneurysms or other lesions is sometimes but not always followed by hemianopsia. Occlusion of the SCA near or beyond the trigeminal root is often followed by infarction, but of a variable size. Occlusion of the AICA proximal to the internal auditory artery is invariably followed by hearing loss, but beyond cranial nerve VIII, the effects are variable. Occlusion of the PICA close to its origin is usually followed by brain stem infarction, but the effects of occlusion further distally are dependent on the size of the PICA (which often has a seesaw relationship with the size of the AICA) and the pial collateral circulation available through the ipsilateral AICA and the contralateral PICA. The safest solution for most patients who require the occlusion of a vessel of this size is revascularization, although this may mean unnecessary revascularizations in some patients. The effects of occlusion of brain stem perforator vessels are also uncertain. Although once thought to be “end arteries,” it is clear that collateral circulation exists [2]. Although it would never be the interest of the surgeon to occlude perforating vessels intentionally, thrombosis of some perforators will frequently occur during the treatment of large, giant, or fusiform aneurysms. In most patients, such presumed thrombosis in the vertebrobasilar segments does not seem to result in stroke, but it can result in stroke and death.
41.4 Learning Curve
The technical difficulties associated with posterior circulation revascularization procedures should not be underestimated. Equally important is the judgment regarding what procedure has the best chance of success and the lowest risk for the patient; the choice for an individual patient may include not doing anything, performing various types of surgical revascularization with aneurysm occlusion, performing an endovascular procedure, or referral to another center (this may not be possible because of logistical and financial reasons). The availability of a good endovascular colleague within the institution, with whom to discuss the pros and cons of surgical, endovascular, or combined procedures, is of great benefit. Neurosurgeons should generally not attempt posterior circulation revascularization procedures before becoming adept with anterior circulation bypass procedures.
41.5 Preoperative Preparation, Anesthesia, and Monitoring
Before performing arterial grafts, we administer aspirin (325 mg orally or 650 mg rectally). During the performance of radial artery or saphenous vein grafting (RAG and SVG, respectively), 3,000 U of heparin are administered intravenously. During temporary arterial occlusion, the blood pressure is increased by 20 % to improve collateral flow in case of unruptured aneurysms and kept at normal levels in ruptured aneurysms. Metabolic brain suppression is induced with propofol to achieve electroencephalographic burst suppression and achieve cessation of synaptic transmission.
41.6 Neuromonitoring
Monitoring of motor-evoked potentials, somatosensory-evoked potentials, brain stem–evoked potentials, and sometimes cranial nerves VII–XII is performed during surgery for complex posterior circulation aneurysms. Cranial-base approaches are used to facilitate posterior circulation revascularization. Upper basilar aneurysms located at or above the level of the dorsum sellae and those involving the PCA or SCA are managed with the frontotemporal/orbitozygomatic [15, 17, 20] approach. The subtemporal/transzygomatic, transpetrous apex approach [3] is used when the aneurysm neck is at the base of the dorsum sellae or even lower. The transpetrosal approach, or one of its variants, is used for aneurysms requiring exposure of the mid-basilar region: transpetrosal-retrolabyrinthine or transpetrosal-partial labyrinthectomy petrous apicectomy (PLPA) with hearing preservation [16], depending on the extent of presigmoid and pre-cerebellar exposure, is required. A combined presigmoid and retrosigmoid or the trans-sigmoid approach may be used with the transpetrosal approach to expose aneurysms and/or arteries that lie in the junctional region between the presigmoid-transpetrosal and retrosigmoid far lateral approaches. A total petrosectomy is used for mid-basilar fusiform or giant aneurysms that cannot be adequately exposed by the PLPA approach [13]. The far lateral retrosigmoid and extreme lateral partial transcondylar or trans-jugular tubercle approaches may be used for approaching many VA, PICA, and AICA aneurysms requiring carotid–VA bypass, extradural–intradural VA bypass, occipital artery (OA)-to-AICA or PICA bypass, and PICA–AICA anastomosis or reimplantation [9]. When an aneurysm is located directly anterior to the lower brain stem, the extreme lateral approach is expanded by the removal of the occipital condyle or jugular tubercle [9]. A midline suboccipital approach may be used for performing a PICA–PICA side-to-side anastomosis [5].
41.7 Vascular Reconstructions
41.7.1 Direct Reconstruction
Direct reconstruction (Fig. 41.2) of cerebral arteries is usually performed after the excision of an aneurysm involving a short segment of a parent vessel. When the artery cannot be mobilized and sutured without tension, then a short interposition graft (Fig. 41.3) can be used.
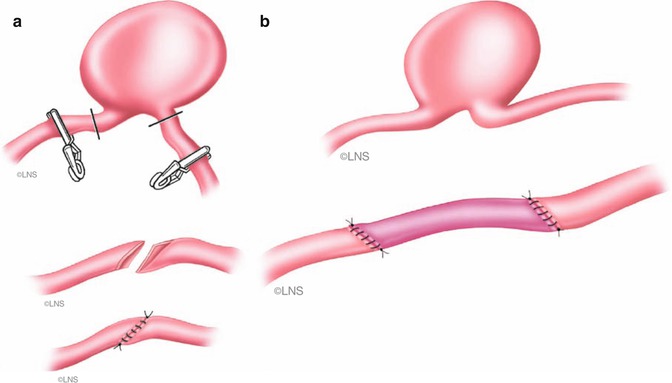
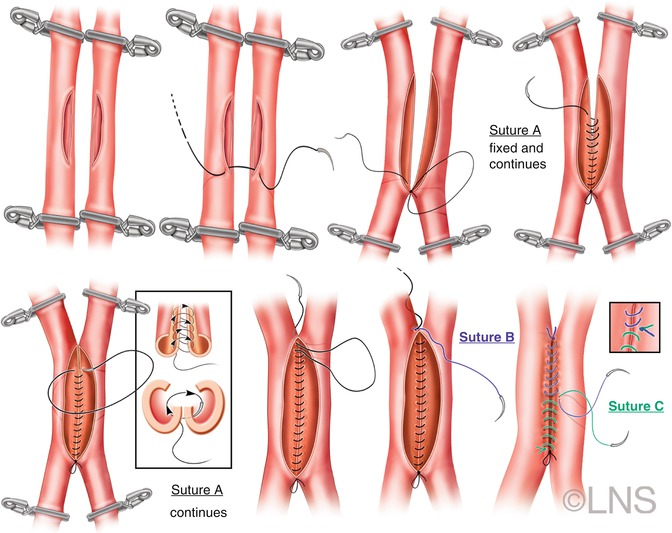

Fig. 41.2
After excision of the aneurysmal sac, arterial end is either sharply sectioned obliquely (a), slightly fish mouthed, and sutured (b) (Courtesy of Laligam N. Sekhar)

Fig. 41.3
Schematic representation of a side-to side anastomosis. Temporary clips are placed on the isolated segments of both arteries, and arteriotomies of equal size are made in both vessels. The diametrically opposite ends are anchored first. Perfect alignment is made with the first two stitches. The posterior wall is anastomosed first with an inside-out technique. Finally, the anterior wall is sutured and the anastomosis completed (Courtesy of Laligam N. Sekhar)
41.7.2 Side-to-Side Anastomosis
Side-to-side anastomosis (Fig. 41.4) of two adjacent arteries, such as AICA–PICA or PICA–PICA, or reimplantation of an artery (e.g., PICA reimplantation into AICA) may be performed if no adequate donor vessel is available for a bypass graft. The vessels must be naturally close to each other or able to be mobilized to lie close to each other, without any tension.
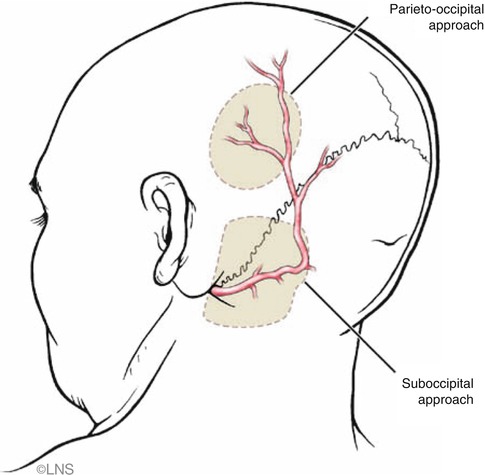

Fig. 41.4
Course of the occipital artery, which is usually more tortuous in the elderly. The sites of parieto-occipital and suboccipital craniotomies are indicated (gray patches) (Courtesy of Laligam N. Sekhar)
41.7.3 Choice of Revascularization Conduits
There are four types of vessels that can be used for surgical revascularization: (1) distant vessels such as the saphenous vein, (2) the radial artery, (3) the OA or STA, and (4) regional vessels such as the AICA, SCA, or PICA (Table 41.1) [6, 10, 12, 14]. The choice of grafting procedure depends on the extent of flow required, the complexity of the operation, and the surgeon’s experience. The VA and (in the very rare case) the BA are good recipient vessels for SVG, but the PCA is less reliable. The transition from a high-flow vessel (SVG) into a low-flow vessel, such as the PCA, results in marked turbulence and may lead to graft occlusion. The senior author has found the RAG to be a good solution for revascularization after VA or BA occlusion because of the better size match to the PCA and the moderate flow rate provided [7, 9, 10, 13]. The major problem in the past was postoperative spasm, which has been mostly solved by the pressure distention technique [8]. The vertebral artery (VA) (V2-3 segment), ECA, or the occipital artery medial to the mastoid can be used as the donor vessel. Smaller arteries, such as the STA, the OA, and regional blood vessels, are very reliable when the vessel being replaced is small, but less so when the BA or VA has to be occluded [7, 12, 14]. The OA and STA are used if they exhibit adequate flow and caliber on angiography and if the recipient vessel is small (2 mm or less) and supplies a territory that is appropriate for the low blood flow rate (20–40 ml/min) typically provided by these donor arteries.
Table 41.1
Surgical revascularization options for posterior circulation aneurysms
PICA fusiform aneurysms | Direct suture, interposition grafting, occipital-to-PICA bypass |
PICA–PICA reanastomosis | |
AICA fusiform aneurysms | Direct suture, interposition grafting, AICA–PICA bypass |
VA fusiform aneurysms | Direct suture, interposition grafting, VA–VA SVG graft |
Low-basilar/vertebrobasilar aneurysms | ECA/VA/MCA to PCA |
Mid-basilar aneurysms | ECA/VA/MCA to PCA/BA, STA–SCA bypass |
Upper basilar aneurysms | ECA/VA/MCA-to-PCA bypass |
41.7.4 Choice of Recipient Vessel
In cases of posterior circulation aneurysm, if the PCA is chosen for the bypass, then the P2 segment of the PCA is selected. It is generally devoid of major perforators. Even if perforators are present, they are not as critical as perforators arising from the P1 segment, and temporary occlusion of this vessel and perforators is remarkably well tolerated. If large perforators arise from P2, we occlude them temporarily with micro-clips during the bypass. The P2 segment is exposed lateral to the midbrain. We use a transpetrosal approach with the division of the tentorium, or a frontotemporal orbitozygomatic approach with tentorial division rather than a subtemporal approach for P2 exposure, since this provides more space to perform the anastomosis. In some patients the P2 may be very high in location, making dissection more difficult. It may also be atherosclerotic. In such a case, a non-sclerotic segment should be selected for bypass. In such cases, the SCA may also be used if it is adequate in caliber. For aneurysms of the VA, the distal VA may be used for bypass when a segment is available for anastomosis. But a bypass to the PCA may be necessary when this vessel is not easily exposed or available. For vertebral-to-PICA aneurysms, the OA is used as a donor vessel. The radial artery is used as a graft when the OA is diseased. In cases of mid-basilar aneurysms, we usually use the PCA as the recipient. However, in one patient (15-year-old girl) the senior author has used the BA distal to the aneurysm as the recipient vessel. This was done under deep hypothermic circulatory arrest after performing a petrosectomy to expose the aneurysm on the previous day. This can be done only in young patients who have good brain plasticity. The SCA may be used as a recipient when the terminal BA is atretic, with two fetal types of PCA.
41.8 OA Exposure
Dissection of the OA is more difficult than the STA because of its deep course (Fig. 41.5). The OA runs horizontally deep to the mastoid tip and digastric muscle, medial to the splenius capitis muscle, medial or lateral to the longissimus capitis muscle, and lateral to the semispinalis capitis muscle. It then perforates the muscular fascia to enter the subcutaneous tissue approximately 2 cm lateral to the midline and turns to run vertically at the level of the superior nuchal line. It has a very tortuous course and gives off multiple muscular branches. An inverted U-shaped incision is made and the skin and subcutaneous flap are reflected. The course of the OA is marked with a Doppler probe. The artery is dissected with the aid of an operating microscope, and bleeding may occur from accompanying veins or muscular arterial branches (Fig. 41.6). Tortuosity makes the dissection difficult. Smaller branches are coagulated away from the main artery and the larger ones ligated. A cuff of periadventitial tissue is left around the artery. For posterior fossa anastomoses, it is adequate to dissect the OA until it penetrates the muscular fascia and turns vertically upward. However, dissection must be done proximally to the digastric groove.
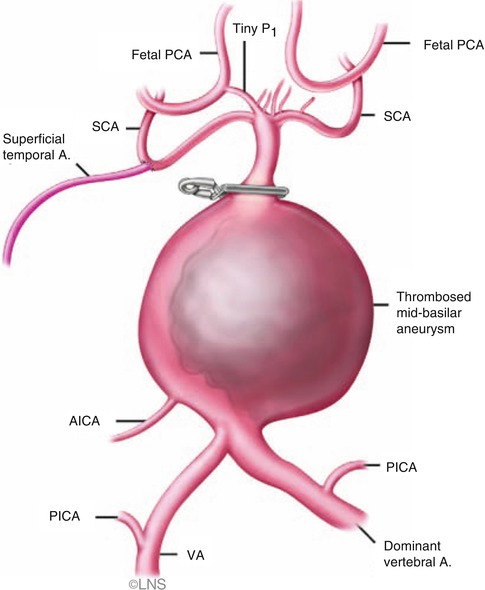
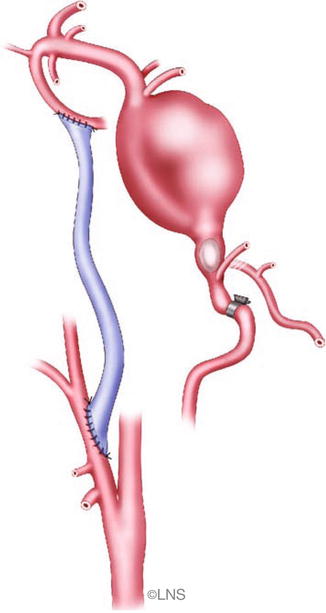

Fig. 41.5
Superficial temporal artery (superficial temporal A.)-to-superior cerebellar artery (SCA) bypass can be done when there is a fetal posterior cerebral artery (PCA) (courtesy of Laligam N. Sekhar). PICA posterior inferior cerebellar artery, AICA anterior inferior cerebellar artery, VA vertebral artery

Fig. 41.6
External carotid artery to P2 segment of the posterior cerebral artery anastomosis using the radial artery or a saphenous vein graft is used to manage a giant mid-basilar aneurysm (Courtesy of Laligam N. Sekhar)
41.9 Extracranial Anastomosis
The proximal anastomosis is performed either in an end-to-end or an end-to-side fashion. An end-to-end anastomosis can always be performed to the external carotid artery (ECA). An end-to-side anastomosis to the internal carotid artery (ICA) can be done with temporary occlusion of the ICA proximal and distal to the anastomosis, if the ECA is not available and the ICA has some intracranial collateral circulation. In cases of the VA, an end-to-side anastomosis is always performed in order to preserve the perfusion of branches proximal to the aneurysm.
41.10 STA–SCA Bypass
This is a rare procedure used for upper basilar aneurysms when there is a fetal PCA or a hypoplastic P1 (Fig. 41.6). A relatively large and long STA is needed. It is dissected as far distally as possible. A lumbar spinal drainage is placed, and the SCA is approached subtemporally with division of the tentorium just posterior to the entrance of cranial nerve IV. A transpetrosal approach may also be utilized. The superior side of the anastomosis is performed first, and then the STA is placed gently under the retractor and the inferior side of the anastomosis is completed. Alternatively, the inferior side can be sutured first by an inside-out suturing technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








