result of hydroxylation of the six-membered aromatic rings, 15% are a result of direct N-glucuronidation at the carbamoyl side chain, and 5% are a result of substitution of the six-membered rings with sulfur-containing groups (42).
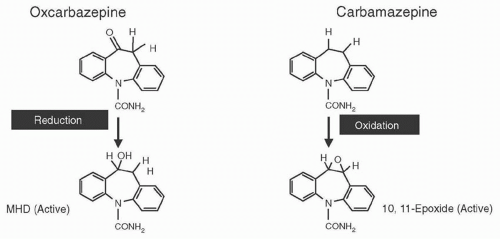 Figure 53.1 Chemical structure and main first-step metabolic pathways of oxcarbazepine and carbamazepine, and their active metabolites, MHD and CBZ-10,11-epoxide (CBZ-E). |
TABLE 53.1 PHARMACOKINETIC PARAMETERS OF CBZ, OXC, AND MHD | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 53.2 PHARMACOKINETIC INTERACTIONS AMONG CBZ, OXC, AND OTHER ANTIEPILEPTIC DRUGS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
detect statistically significant differences (72). Because of the above-mentioned data, CBZ has been considered a first-line AED for the treatment of partial and secondarily generalized tonic-clonic seizures, and is used as an active control in trials of all new compounds.
2 to 2.5 per million. Aplastic anemia occurs with CBZ exposure in 5.1 per million (1 per 200,000) (88,114).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






