Figure 32.1. Histopathologic and imaging stages of the T. solium cysticercus in the brain parenchyma. A: Histologic features of the vesicular stage with a well-defined cysticercus including its extensive canalicular network and no surrounding inflammation. B: T1-weighted MRI showing a cyst in its active (vesicular) stage with a well-defined eccentric scolex and no surrounding edema. C: Unenhanced CT showing multiple active vesicular cysts. D: Histologic features of the colloidal stage (corresponding to the degenerating or encephalitic/transitional on imaging) with surrounding inflammation. E: Gadolinium-enhanced MRI demonstrating a ring-enhancing lesion in the brain parenchyma corresponding to the degenerating (transitional or encephalitic) stage. F: Contrast-enhanced CT showing a similar ring-enhancing lesion probably corresponding to the granular–nodular pathologic stage. G: Histologic depiction of the fibrocalcific parasite corresponding to the dead or inactive parasite with little surrounding inflammation. H: CT appearance of inactive, fibrocalcific nodule. I: CT demonstrating multiple NCC, some live, active and others calcified, thus multiple stages at any given point of time. (A, D, and G, Courtesy of Alfonso Escobar, UNAM, Mexico; B, C, and I, from Singh G, Prabhakar S. Taenia solium Cysticercosis: From Basic to Clinical Science. Oxon, UK: CAB International, with permission.)
Symptoms of cysticercosis are almost always the result of brain infestation and depend on the location, stage, and number of cysts in the brain. Seizures, which occur in nearly 80% of cases of NCC, are most characteristic of parenchymal location (16). The number of cysts in the brain also has a bearing on the clinical presentation. When cysts are extremely frequent, especially in 100s, the clinical presentation is dominated by features of raised intracranial pressure, dementia, and focal neurologic deficits (17). Conversely, patients with solitary cysticercus granuloma or a small number of cysts present with few seizures, and these are usually easily controlled with antiepileptic drugs (AEDs) (18).
Other CNS Infections
Several CNS infections are notable for their association with seizures and epilepsy. CNS tuberculosis, especially when presenting with cerebral tuberculoma, bacterial brain abscess, fungal infections including cryptococcal meningitis, and subacute sclerosing panencephalitis deserve mention due to a notable frequency of both early and late unprovoked seizures (19).
BURDEN OF CNS INFECTIONS
The epidemiology of many CNS infectious disorders has changed in the recent past, and these changes have perhaps impacted their association with epilepsy. For instance, the introduction of H. influenzae type b (Hib) and S. pneumoniae conjugate vaccines has led to a decline in the incidence of bacterial meningitis in the United States from 2/100,000 in the 1990s to 1.38/100,000 in 2007 (20). Likewise, attempts to control transmission of N. meningitides meningitis and malaria in sub-Saharan Africa and of cysticercosis in South and Central Americas have led to a prefatory decline in the incidence of these disorders. At the same time, land development and increasing human traffic between endemic resource-poor countries and high-income countries have also contributed to the changing epidemiology of CNS infections. For example in the United States where T. solium was once considered eradicated, large numbers of NCC cases have been reported from states with the largest volume of Latino immigrants, that is, California, Texas, and Oregon (21). In California alone, where NCC is a reportable disease, there occurred 805 hospitalizations with a discharge diagnosis of NCC in 2009, amounting to 304 hospitalized people (estimated incidence: 0.8/100,000) with six deaths (22). Nearly 85% of those hospitalized were Latinos.
An estimated 19,000 hospitalizations and 1400 deaths with a diagnosis of viral encephalitis take place each year in the Unites States (4). Elsewhere, the burden has not been precisely investigated but is probably enormous. About 30,000 to 50,000 cases of Japanese B encephalitis are recorded annually from South and Southeast Asia (23). In sub-Saharan Africa, where over 80% of malaria episodes are recorded, an estimated 600,000 cases of cerebral malaria occur each year among children, of whom 100,000 (20%) die and 20,000 are left with serious neurologic sequelae beyond 6 months (24,25). Likewise, in this region, the estimated annual incidence of meningococcus meningitis is 20/100,000 population, amounting to 500,000 cases each year (26). The estimated burden of NCC in Latin America alone is 400,000 among a population of 75 million (27). A high prevalence of NCC, albeit of lesser magnitude, has also lately been documented in South Asia and sub-Saharan Africa (28,29).
ASSOCIATION BETWEEN CNS INFECTIONS AND EPILEPSY
Longitudinal data representing the magnitude of risk of epilepsy following a CNS infection episode are available for the Olmsted County community in Minnesota (30). In this community, the 20-year risk of unprovoked seizures was 22% following an episode of encephalitis with early (<7 days) seizures, 10% in the absence of early seizures, 13% after an episode of bacterial meningitis with early seizures, and only 2% after meningitis in the absence of early seizures. Aseptic meningitis does not appear to increase the risk of late unprovoked seizures. In incidence studies from western high-income countries, CNS infections constitute the putative etiology in 5% of new-onset epilepsies (31). Community-based figures on incidence in resource-poor countries are not available but numbers are probably even higher.
Lately, the association between several infectious disorders and epilepsy has been studied in endemically specified geographic regions. Both in Latin America and India, a number of case–control studies using serology or brain imaging as evidence of infection have demonstrated a significant association between cysticercus infection and epilepsy (32–34). These studies have consistently shown that NCC is a risk factor for roughly one-third of active epilepsies in endemic communities (32). Likewise, studies using standard case definitions for cerebral malaria from Kenya, Mali, and Gabon have established a moderate association between the cerebral malaria and epilepsy (35–37). In contrast with these association studies, in which causality between the infection and epilepsy appears plausible, studies in disparate locations (including Burundi, Bolivia, and Italy) have also suggested an association between exposure to Toxocara canis (dog roundworm) and epilepsy (38–40). It might be clarified here that the demonstration of an association does not imply causality between T. canis infection and epilepsy. For one reason, brain infection with T. canis has rarely been documented either by imaging or pathology. For another, it is possible that a confounding variable, for example, socioeconomic deprivation, which is a risk factor for both T. canis infection and epilepsy, might account for the association. Moreover, the findings of an association have not been confirmed in other regions (33).
SEIZURES IN RELATION TO CNS INFECTIONS (PROVOKED VERSUS UNPROVOKED)
Seizures are common during the active (often acute) phase of CNS infections and are referred to as “early,” “acute asymptomatic,” or “provoked” seizures. In the Olmsted County community, 15% of all acute symptomatic seizures were associated with CNS infections, yielding an age-adjusted incidence of 5.2/100,00 person-years (41). Early seizures occur in 33% of the cases with bacterial meningitis, 40% to 60% of HSV encephalitis, and in up to 67% of Japanese B encephalitis (1,42).
In many acute CNS infections, seizures may occur as subtle nonconvulsive seizures or as convulsive status epilepticus. Both convulsive and nonconvulsive status epilepticus have been documented in bacterial meningitis, HSV and Japanese B encephalitides, and cerebral malaria (43–45). The documentation of status epilepticus during the acute infection episode correlates with increased intracranial pressure and poor outcome (43,45).
The structural and pathophysiologic correlates of seizures during acute infections vary and include focal hemorrhagic necrosis in the cerebral cortex (HSV-1 and other encephalitides), vasculitis and infarcts (bacterial meningitis), cerebral abscesses (pyogenic, tubercular and fungal), the release of interleukins, anti-infective medications (e.g., imipenem, flouroquinones, and isoniazid), raised intracranial pressure and disruption of the blood–brain barrier (46).
The definition of acute symptomatic seizures was recently revised (47). In the context of CNS infections, seizures might be considered as acute symptomatic (or provoked) if these occur during the phase of active infection. The categorization of acute symptomatic or provoked seizures as early seizures is easily understood in acute CNS infections (e.g., bacterial meningitis, viral encephalitis, and cerebral malaria) but becomes problematic in the case of chronic CNS infections such as NCC. It has been suggested that seizures occurring in relation to the degenerating stages (colloidal vesicular or granulonodular stages) of NCC are provoked, while those occurring in relation to the inactive (or fibrocalcified) stage are unprovoked (48). The degenerating stages are characterized by a breakdown of the blood–brain barrier and host inflammatory response surrounding the cerebral cysticercus. The cellular and molecular phenomena associated with inflammatory degeneration probably provoke seizures (49). Even so, recent follow-up studies of patients with calcified cerebral cysticerci have shown that roughly 50% continue to have seizures. Of these, in 50% without ongoing seizures, the occurrence of seizures is related to an unpredictable and sporadic release of antigenic material from the calcified cysticercus remnants, thereby engendering breakdown of the blood–brain barrier and inflammatory response in the surrounding cerebral parenchyma (50). These episodes are characterized by contrast enhancement and surrounding edema around the calcified cysticercus (Fig. 32.2). Hence, these unpredictable events underscore the problems in categorizing seizures in relation to NCC as “acute symptomatic” or “unprovoked.”
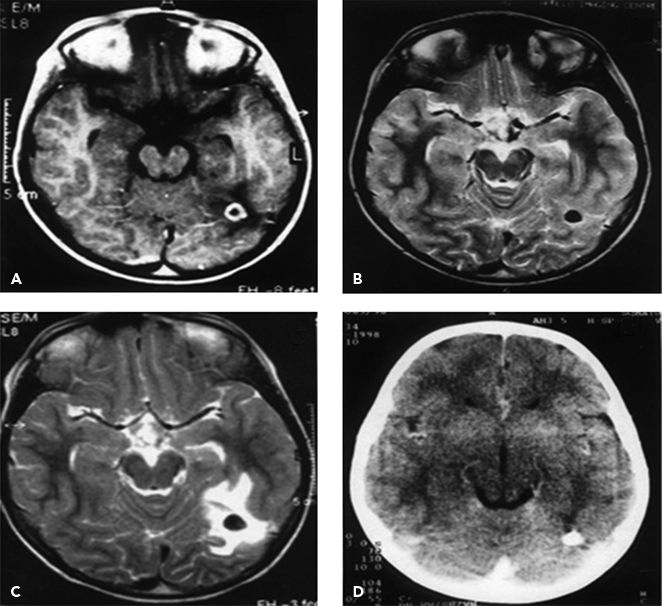
Figure 32.2. Resolution of a cysticercus granuloma and the subsequent reappearance and resolution of perilesional edema. A: Initial gadolinium-enhanced MRI in a patient with recent seizures showing a single annular-enhancing lesion. B: Follow-up T2-MRI 6 months later showing resolution with calcification. C: T2-MRI performed another 14 months later, when the patient developed seizure recurrence, showing perilesional edema around the calcific nodule. D: CT scan performed another 6 months later showing resolution of the edema around the calcific nodule. (From Singh G, Prabhakar S. Taenia solium Cysticercosis: From Basic to Clinical Science. Oxon, UK: CAB International, with permission.)
The occurrence of early seizures during the CNS infection episode increases the risk of late unprovoked seizures (amounting to epilepsy if recurrent) (30). This risk might be as high as 24% when the infection episode associated with early seizures leaves behind a structural residue in the cerebral parenchyma (51). In addition, the risk of a late seizure is particularly high when the infection episode is accompanied by status epilepticus instead of an uncomplicated seizure.
TREATMENT CONSIDERATIONS
There are few data in the form of clinical trials and observational follow-up studies to support the choice and duration of AED treatment in people with epilepsy associated with CNS infections. Treatment policies might therefore follow guidelines for treatment of symptomatic epilepsies associated with structural cerebral lesion(s). Two additional issues merit consideration. One is the putative effect of treatment interventions in relation to the infectious disorder on the subsequent risk of development and outcome of epilepsy. The other is the range of drug interactions between AEDs and medications used to treat the infectious disorder.
The treatment of CNS infections comprises antibiotics for bacterial meningitis, antivirals for viral encephalitis, antimalarial medications for cerebral malaria, and antihelminthic agents (albendazole and praziquantel) for NCC. Other agents, for example, corticosteroids in bacterial meningitis and NCC and AEDs during the active phase of the infectious disorder, might also be administered. When administered early during the course of bacterial meningitis, corticosteroids reduce the risk of certain neurologic sequelae (e.g., sensorineural deafness), but the effect of the risk of development of unprovoked seizures has not been studied. The administration of AEDs during the acute infection episode (in bacterial meningitis, viral encephalitis, and cerebral malaria) does not appear to influence the risk of development of subsequent epilepsy.
Treatment of NCC comprises of antihelminthic agents (albendazole and praziquantel) with or without corticosteroids. A randomized, placebo-controlled trial of albendazole in people with live NCC demonstrated significantly improved clearance of the parasite with treatment (52). However, the benefits of administration of albendazole with respect to seizure outcome were less certain. Overall, there was no significant difference in the number of subjects in the treated and placebo groups who remained seizure free over a 30-month follow-up period (52). Many more controlled trials have studied the effects of albendazole and corticosteroids in people with NCC. Meta-analyses of trials of antihelminthic treatments and corticosteroids alone in solitary cysticercus granuloma have suggested that while antihelminthic treatment is associated with greater chances of seizure freedom, treatment with a short course of corticosteroid alone does not influence seizure recurrence rates (53).
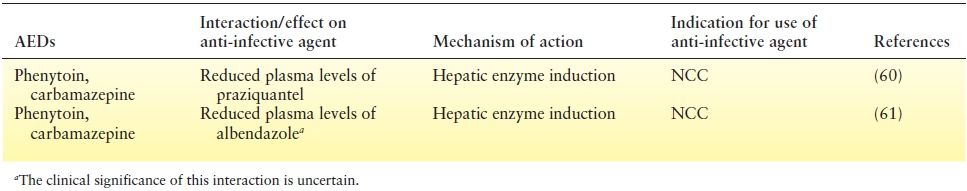
The other issue of concern is the range of drug interactions between AEDs and anti-infective medications used in the treatment of CNS infections. These interactions (Tables 32.1 and Table 32.2) might adversely impact not only the treatment of the infectious disorder leading to reduced chances of a cure but also epilepsy resulting either inadequate seizure control due to reduced AED levels or conversely, AED toxicity.
Table 32.1 Effect of Anti-Infective Agents on AEDs
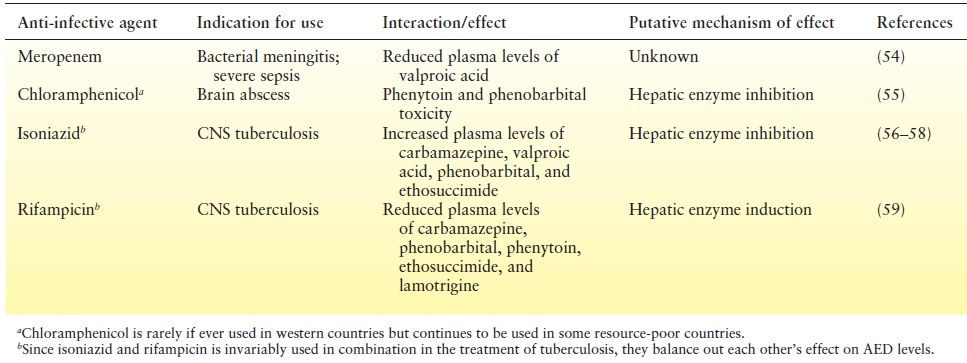
Table 32.2 Effect of Aeds on Plasma Levels of Anti-Infective Agents
SURGICALLY REMEDIABLE EPILEPSY IN RELATION TO CNS INFECTIONS
Heretofore, the prognosis for seizure control with standard single AEDs in disorders such as NCC was believed to be good. Furthermore, antecedent CNS infection (e.g., meningitis and encephalitis) conferred a poor prognosis following surgery for medically intractable epilepsy owing to the widespread cerebral damage sustained thereof. Thus both, the development of refractoriness to AED treatment in NCC and the potential for benefit following surgical treatment for epilepsy following meningitis and encephalitis, were not well appreciated, largely due to absence of systematic follow-up studies of CNS infections.
MEDICALLY REFRACTORY EPILEPSY IN ASSOCIATION WITH CALCIFIED CEREBRAL CYSTICERCI
Rare cases with AED-refractory seizures shown to arise from the vicinity of a calcified cerebral cysticercus and having benefited from surgical lesionectomy alone have been documented (62,63). Perhaps more common is the occurrence of mesial temporal lobe epilepsy with hippocampal sclerosis and a calcified cysticercus located within or in its close proximity (62,64). The hippocampus is not a preferred location for the cysticercus. In general, the cysts commonly lodge in the frontal and parietal lobes, in keeping with the differential proportion of blood supply delivered to various lobes of the brain (65). However, when a cyst does lodge within or close to the hippocampus, the likelihood of developing recurrent and poorly controlled seizures appears to be high, partly due to vulnerability of the hippocampus to insults and partly due to presence of neuronal circuitry conducive to epileptogenesis. In this situation, the prognosis for seizure control following standard temporal resections that include the calcified cysticercus is excellent (62).
A distinct clinical scenario is the finding of one or more calcified cerebral cysticercus lesions in association with unilateral hippocampal sclerosis upon imaging studies in people with medically refractory mesial temporal lobe epilepsy. Calcified cysticerci might be identified on imaging studies in a sizeable proportion of completely asymptomatic individuals in communities that are endemic for T. solium infestation (66). Hence, it is not surprising that a proportion of patients undergoing presurgical evaluation in epilepsy centers located in endemic regions (e.g., Brazil or India) demonstrate one or more calcified cysticerci on imaging studies. This might imply that the calcified cysticerci are detected as mere coincidental findings on imaging studies in people with hippocampal sclerosis.
A cross-sectional study at a Brazilian epilepsy center found a significant association between calcified cysticerci and hippocampal sclerosis but not other substrates for surgically remediable epilepsy (e.g., tumors, developmental malformations, etc.) (67). A moot point relating to the implied association between the calcified cysticercus and hippocampal sclerosis is whether NCC interacts with the development of the hippocampal pathology (e.g., as an antecedent or as a result of kindling initiated by NCC-associated seizures) or if the occurrence of NCC and hippocampal sclerosis is purely coincidental. Another controversial area is whether the interaction influences the choice of surgical approaches to medically intractable epilepsy with hippocampal sclerosis and calcified NCC. A Brazilian report of 32 patients with hippocampal sclerosis in association with calcified NCC described Engel Class I seizure outcome in 82% following standard anteromesial temporal resection alone (68). Conversely, in a report from India, seizure freedom was observed in only one out of four patients following temporal resection alone (62). In another two patients in whom independent seizures were demonstrated to arise from both the hippocampus and the calcified NCC, complete seizure freedom was obtained following temporal resection along with lesionectomy (resection of the calcified NCC) due to suspected dual pathology. Although these data are preliminary, it is recommended that the surgical approach to hippocampal sclerosis associated with calcified NCC be decided on a case-by-case basis, keeping in mind that seizures might arise from either the hippocampus or from both, the hippocampus and the calcified NCC.
MEDICALLY INTRACTABLE EPILEPSY WITH ANTECEDENT MENINGITIS AND ENCEPHALITIS
The development of medically intractable epilepsy with antecedent meningitis or encephalitis can take place in one of the following three forms:
1. Unilateral hippocampal sclerosis with antecedent meningitis or encephalitis: The antecedent infection (more commonly meningitis and less commonly encephalitis) usually occurs before the age of five years (69). Mesial temporal lobe epilepsy (Fig. 32.3) follows the infection episode after a latent period of several years (70,71). The prognosis for seizure control following standard temporal lobe resections is excellent (72).
2. Bilateral hippocampal sclerosis with antecedent meningitis or encephalitis: Although there is a potential for independent seizure origin from either or both mesial temporal lobes, epilepsy surgery might still be a feasible option provided that a sufficient number of seizures have been recorded and are demonstrated to arise from only one side (69,73).
3. Neocortical epilepsies with antecedent meningitis or encephalitis: The infection episode (most often encephalitis) takes place after the age of five years (69). The epilepsy has an onset soon after the infection episode and has a catastrophic evolution (70). The seizure outcome following neocortical resection is not good. It is unclear whether the poor outcome is related to inadequate localization of the epileptogenic zone or to the presence of multiple epileptogenic foci.
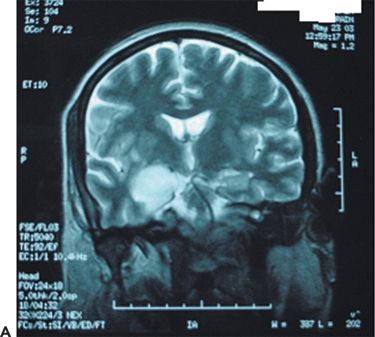
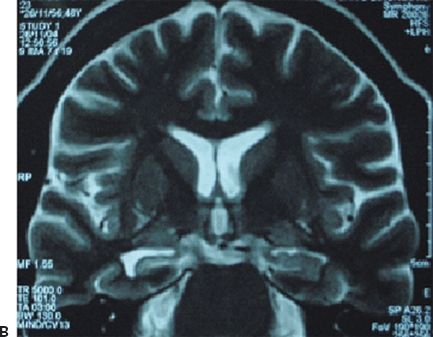
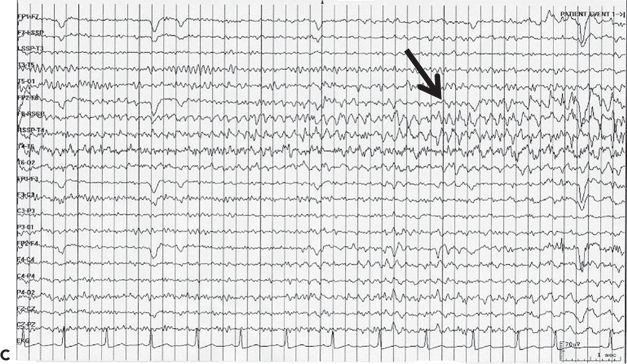
Figure 32.3. Postencephalitic epilepsy. A: T2-weighted coronal MRI demonstrating predominantly right mesial temporal hyperintensity and swelling during presumed HSV-1 encephalitis. B: Follow-up coronal T2 MRI of the same patient 2 years later revealed sclerosis within an atrophic right hippocampus. C: Ictal EEG in the same patient demonstrating an electrographic seizure origin from the right temporal lobe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








