Chapter 22 Cerebral Cortex
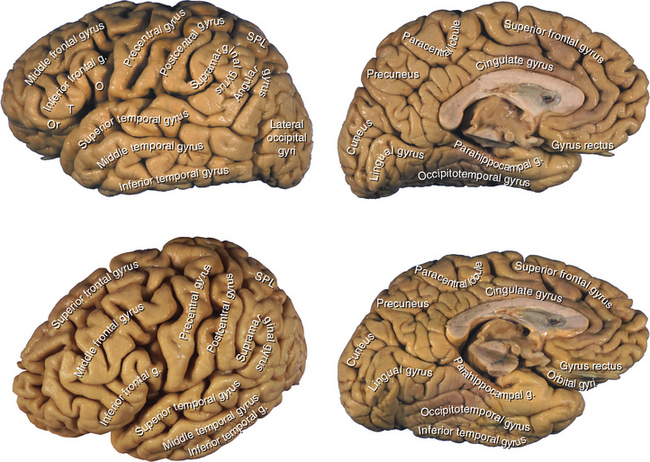
The cerebral cortex is a sheet of neurons and their interconnections, about 1800 cm2 (2 ft2) in area, that plates the corrugated surface of the cerebral hemispheres in a layer just a few millimeters thick. This thin layer of gray matter accounts for nearly half the weight of the brain and is estimated to contain about 25 billion neurons, interconnected by more than 100,000 km of axons receiving an incredible 1014 synapses. The corrugation into gyri and sulci, a mechanism for increasing the area of cortex, is reasonably constant in its major features (Fig. 22-1).
One of the more striking changes that has occurred in the course of the evolution of vertebrate brains is the tremendous increase in the relative size of the cerebral hemispheres and the even greater increase in the area of cerebral cortex on their surfaces. One inference drawn from this fact (and supported by abundant clinical and functional imaging evidence) is that the cerebral cortex has a critical role in the abilities and activities that reach their highest level of development in humans (or, in some cases, are unique in humans). Obvious examples are language and abstract thinking. These are, of course, not the only functions of the cerebral cortex; basic aspects of perception, movement, and adaptive response to the outside world also depend on it.
Most Cerebral Cortex Is Neocortex
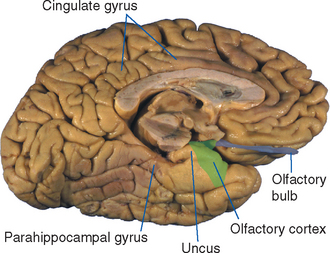
Cerebral cortex does not have the same structure everywhere. Almost all the cortex that can be seen on the outside of the brain is of a type called neocortex, neo referring to the idea that it first appeared fairly late in vertebrate evolution. Reptiles have cerebral cortex, but all of it consists of three-layered types that continue in humans as paleocortex and archicortex, named in reference to their supposedly more ancient origins. * Paleocortex covers some restricted parts of the base of the telencephalon (Fig. 22-2), and most of the hippocampus is archicortex. Neocortex has a different structure, described shortly, and develops interposed between the paleocortex and archicortex, separated from them by cortical transition zones with intermediate structures. Some mammals have relatively little neocortex, but it expands greatly in primates, accounting for about 95% of the total cortical area in humans. This expansion causes the apparent rotation of the cerebral hemispheres into their characteristic C shape, with paleocortex and archicortex at the two ends of the C (see Fig. 2-13).
All neocortical areas go through a period during development in which they have a six-layered structure. As discussed shortly, this layered appearance is altered in some areas of the adult brain, but in view of its uniform early development, neocortex is also referred to as homogenetic cortex or isocortex. In contrast, paleocortex and archicortex never go through such a six-layered stage and are referred to collectively as heterogenetic cortex or allocortex (from the Greek word allo, meaning “other”). The hippocampus is a component of the limbic system (see Chapters 23 and 24), and paleocortex, which develops in conjunction with the olfactory system (see Chapter 13), is closely interconnected with limbic structures; the remainder of this chapter deals with neocortex.
Pyramidal Cells Are the Most Numerous Neocortical Neurons
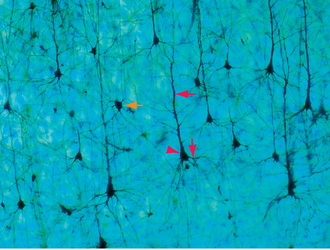
Pyramidal cells, the most numerous neurons of the neocortex, are named for their shape (Fig. 22-3). These cells have a conical cell body from which a series of spine-studded dendrites emerge—a long apical dendrite that leaves the “top” of each cell and ascends vertically toward the cortical surface, and a series of basal dendrites that emerge from nearer the base of the cell and spread out horizontally. Pyramidal cells range in size from less than10 μm in diameter all the way up to the giant pyramidal cells (Betz cells) of the motor cortex, which are among the largest neurons in the central nervous system (CNS), some measuring more than 100 μm from their base to the beginning of the apical dendrite. Most or all pyramidal cells have long axons that leave the cortex to reach either other cortical areas or various subcortical sites, where they make excitatory (glutamate) synapses. The remaining cortical neurons are spoken of collectively as nonpyramidal cells. Many are small (often less than 10 μm), multipolar stellate (or granule) cells, but a variety of other types and sizes have been described (Fig. 22-4). With few exceptions, nonpyramidal cells have short axons that remain within the cortex. One kind of nonpyramidal cell has spine-covered dendrites, receives inputs from the thalamus, and makes excitatory (glutamate) synapses on nearby neurons; most or all of the others make inhibitory (γ-aminobutyric acid [GABA]) synapses on their targets. Hence pyramidal cells are the principal output neurons of the neocortex, and nonpyramidal cells are the principal interneurons.
The dendritic spines of pyramidal cells (see Fig. 1-4E) are preferential sites of excitatory synaptic contacts and have been the source of considerable interest and some mystery as well. They are not merely a device for increasing dendritic surface area, because the portions of a dendrite located between spines are sparsely populated with synaptic contacts. It has been suggested that dendritic spines may be the sites of synapses that are selectively modified as a result of learning, because small changes in the geometry of a spine can cause relatively large changes in its electrical or diffusional properties and therefore in the efficacy of that synapse. Certain cases of mental retardation are accompanied by faulty development of dendritic spines, but which is cause and which is effect (if either) is not known. Certainly, however, the most remarkable change that occurs in the cortex after birth is the tremendous expansion of the dendritic trees of its neurons and a parallel increase in the number of dendritic spines. It should be noted that spines are not unique to cortical pyramidal cells; they are also found on the dendrites of some other neurons, such as Purkinje cells (see Fig. 8-16) and many striatal neurons.
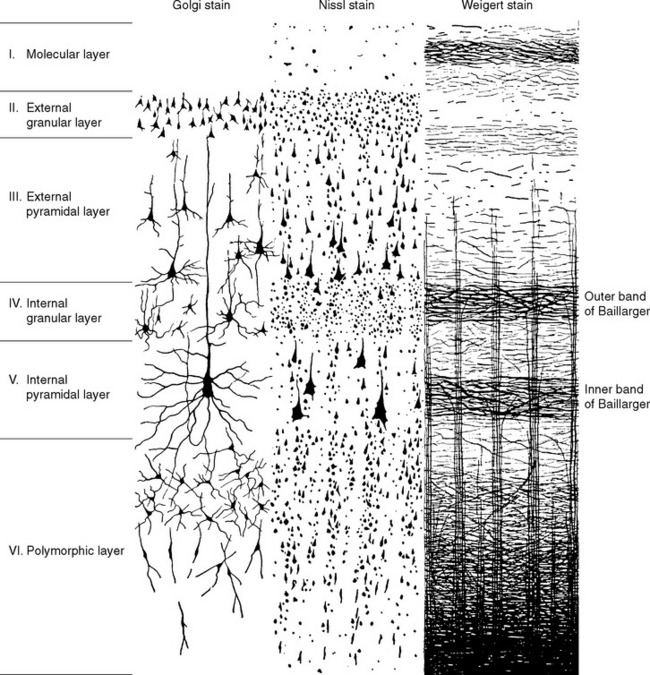
Neocortex Has Six Layers
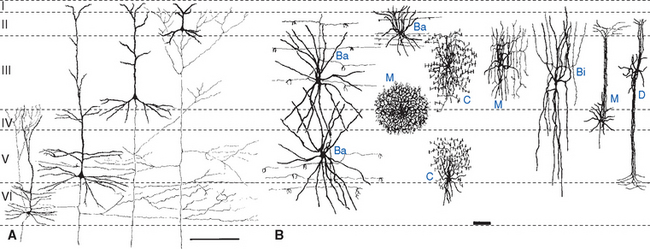
The cells of the neocortex are arranged in a series of six layers, more apparent in some areas than in others. Just as in the case of cerebellar cortex (see Figs. 20-9 and 20-10), the most superficial layer is a cell-poor molecular layer. The deepest neocortical layer is the polymorphic (or multiform) layer, which is populated largely by fusiform-shaped modified pyramidal cells. In between the molecular and polymorphic layers are four layers alternately populated mostly by small cells or mostly by large pyramidal cells. The layers are commonly designated by roman numerals and by names, as indicated in Figure 22-5. Myelin staining reveals vertically oriented bundles of cortical afferents and efferents, as well as horizontal bands through which these fibers and intracortical axons spread. Two particularly prominent horizontal bands are contained in layers IV and V and are called, respectively, the outer and inner bands of Baillarger.
Neocortex does not have the same striking regularity as cerebellar cortex; its six cell layers are not equally prominent everywhere. Areas that give rise to many long axons (e.g., the motor cortex) would be expected to have numerous large pyramidal cells, and this is indeed the case (Fig. 22-6A). In these areas, nonpyramidal cells appear minor by comparison, and layers II through V are dominated by large pyramidal cells to the extent that individual layers are no longer obvious. Because of the apparent lack of stellate (granule) cells, such cortex is called agranular. In contrast, primary sensory areas project mainly to adjacent cortical areas and do not give rise to many long axons. They have a corresponding dearth of large pyramidal cells; here too, layers II through V look like one continuous layer, but in this case they are dominated by small cells (both pyramidal and nonpyramidal; Fig. 22-6A). Such cortex is therefore called granular cortex or koniocortex (from the Greek word konia, meaning “dust,” referring to the numerous tiny cells). There is a continuum of structural types ranging from thick (up to 4.5 mm) agranular cortex to thin (as little as 1.5 mm) granular cortex (Fig. 22-7). The intermediate kinds, in which the six neocortical layers are relatively distinct, are called homotypical cortices (as opposed to granular and agranular cortices, which are collectively called heterotypical [Fig. 22-6B]).
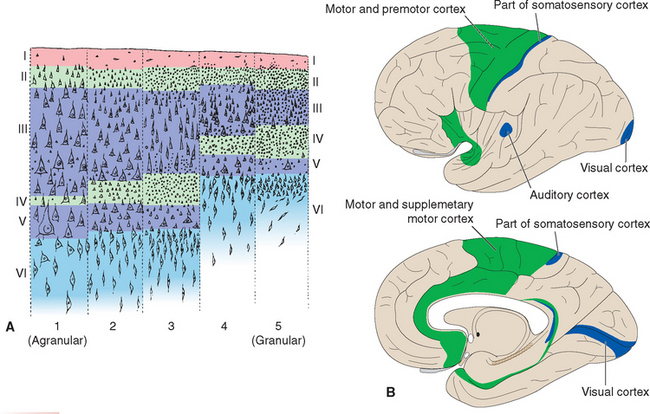
Figure 22-6 A, Different types of neocortex. At the two extremes are the heterotypical cortices: agranular cortex (1) dominated by large pyramidal cells, and granular cortex (5, koniocortex) dominated by small cells. Areas with intermediate structures in which six layers can be discerned more clearly are homotypical and were divided into three types by von Economo: 2, frontal type; 3, parietal type; 4, polar type; B, Distribution of heterotypical cortex. The lateral view, above, is drawn as though the lateral sulcus had been pried open, exposing the insula. Agranular cortex (green) is found primarily in motor areas, granular cortex (blue) primarily in sensory areas (compare with Fig. 22-13).
(Modified from von Economo C: The cytoarchitectonics of the human cerebral cortex, Oxford, 1929, Oxford University Press.)
The differences among cortical areas are to some extent more apparent than real. Beneath a square millimeter of any area of mammalian cortex, whether from a hamster or a human, lie approximately the same number of neurons (roughly 100,000). The major exception is the binocular portion of the primary visual cortex of primates, where the neurons are packed somewhat more densely. About 80% of the neurons in all cortical areas are pyramidal cells. Hence 80% of the neurons in granular cortex are very small pyramidal cells. Different cortical areas have different appearances and functions because of the relative sizes of the cell types, the complexities of their dendritic trees, and the patterns of their connections. This fundamental similarity of all cortical areas is one aspect of the notion, discussed a little later in this chapter, that the cerebral cortex may be a large array of small, repeated functional units.
Different Neocortical Layers Have Distinctive Connections
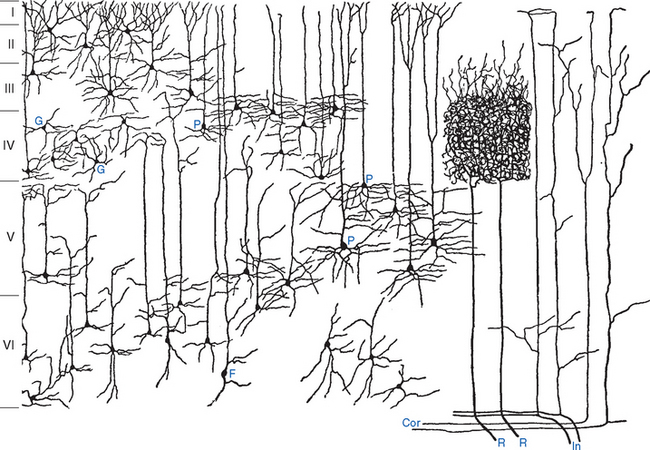
Afferents to the cortex can come from only two general places: other cortical areas and subcortical sites. Afferents from other cortical sites, by far the majority, may arise in the same hemisphere (association fibers) or in the contralateral hemisphere (commissural fibers). The predominant subcortical source of afferents is the thalamus, and its pattern of projections is described in Chapter 16. Other subcortical sites, such as the locus ceruleus and other chemically coded nuclei, also provide modulatory afferents to the cortex (see Chapter 11 and later in this chapter).
These various types of incoming fibers ramify within the cortex in different patterns (Fig. 22-8). For example, afferents from thalamic relay nuclei end primarily in the middle layers, as in the dense arborizations in layer IV of fibers from sensory relay nuclei; * fibers from other thalamic nuclei and from other cortical areas ascend vertically and terminate diffusely along their course in distinctive patterns (e.g., those from intralaminar nuclei mostly in layer VI, and those from other cortical areas mostly in layers II and III).
The Corpus Callosum and Anterior Commissure Interconnect the Two Cerebral Hemispheres
Most efferents to the cortex of the contralateral hemisphere pass through the corpus callosum, as described later in this chapter (see Fig. 22-28). Those interconnecting parts of the temporal lobes (particularly inferior parts) traverse the anterior commissure, along with crossing fibers from the anterior olfactory nucleus (see Figs. 13-17 and 13-18).
Association Bundles Interconnect Areas within Each Cerebral Hemisphere
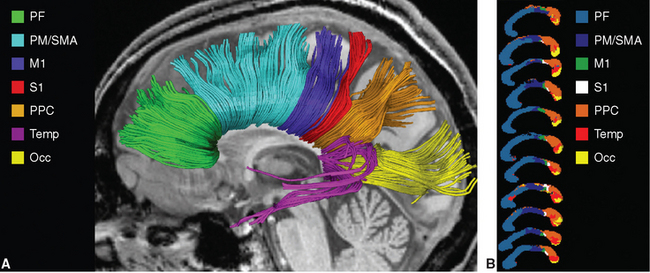
Efferents to ipsilateral cortical areas come in all lengths, from very short ones that never leave the cortex, to U-shaped fibers that dip under one sulcus to reach the next gyrus, to longer association fibers that travel to a different lobe; collectively, they account for a large majority of the axons in the white matter of each hemisphere. The longer fibers collect into reasonably well-defined bundles that can be found by gross dissection (Fig. 22-9A) and now by diffusion tensor imaging (Fig. 22-9B to D). The most prominent of these association bundles are the superior longitudinal fasciculus, the superior and inferior occipitofrontal fasciculi, and the cingulum. The superior longitudinal fasciculus (also called the arcuate fasciculus) sweeps along in a great arc above the insula between the frontal lobe and posterior portions of the hemisphere, where it fans out among the parietal, occipital, and temporal lobes. The superior occipitofrontal fasciculus, as its name implies, runs between the frontal lobe and superior parts of the parietal and occipital lobes. It travels parallel to the corpus callosum and, for much of its course, is located between the corpus callosum and the caudate nucleus. Here it lies adjacent to the subcallosal fasciculus, a pale-staining bundle of fibers on their way from several cortical areas to the caudate nucleus (see Fig. 19-1). The inferior occipitofrontal fasciculus passes below the insula between the frontal and occipital lobes, traversing the temporal lobe along the way. Its fibers fan out at both ends of the fasciculus, and those at its anterior end lie adjacent to the uncinate fasciculus (from the Latin uncus, meaning “hook”), an association bundle that hooks around the margin of the lateral sulcus to interconnect the orbital cortex and anterior temporal cortex. Finally, the cingulum courses within the cingulate gyrus and continues around within the parahippocampal gyrus to nearly complete a circle. None of these association bundles is a discrete, point-to-point pathway from one place to another; rather, fibers travel in both directions and enter and leave each pathway all along its course. The inferior occipitofrontal fasciculus provides a good example of this. Few of its fibers actually extend all the way between the occipital and frontal lobes. Rather, those in the posterior part of this fasciculus interconnect occipital and temporal areas and are often considered separately as the inferior longitudinal fasciculus; anterior fibers travel through inferior parts of the extreme capsule to interconnect the superior temporal gyrus, the insula, and orbital and prefrontal cortex.
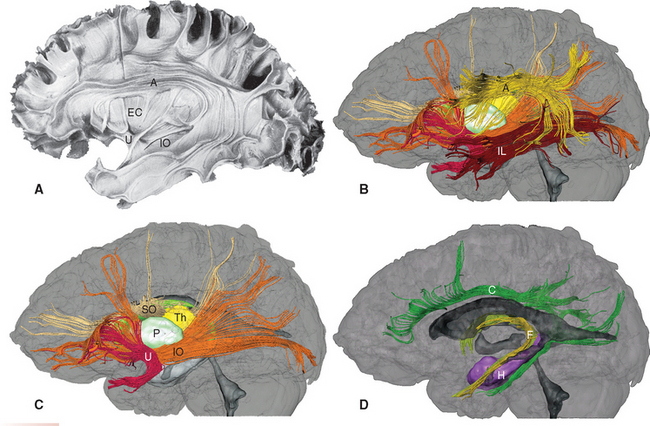
Figure 22-9 Long association bundles of the left cerebral hemisphere, as seen by partially dissecting a hemisphere (A) and in diffusion tensor images (B to D). A, arcuate (superior longitudinal) fasciculus; C, cingulum; EC, extreme capsule; F, fornix and stria terminalis (see Chapter 23); H, hippocampus; IL, inferior longitudinal fasciculus; IO, inferior occipitofrontal fasciculus; P, putamen; SO, superior occipitofrontal fasciculus (combined with the subcallosal fasciculus adjacent to it); Th, thalamus; U, uncinate fasciculus.
(A, from Ludwig E, Klingler J: Atlas cerebri humani, Boston, 1956, Little, Brown. B to D, from Mori S et al: MRI atlas of human white matter, Amsterdam, 2005, Elsevier.)
Neocortex Also Has a Columnar Organization
In spite of the fact that the cortex is horizontally laminated, one gets the strong impression that there is also a vertical organization (“vertical” meaning perpendicular to the surface). Apical dendrites of pyramidal cells have vertical courses, as do afferents to the cortex and the axons of some intracortical cells (Fig. 22-8); even the cell bodies of cortical neurons often look as though they are arranged in vertical columns (Fig. 22-5). Both physiological and anatomical studies have shown that this is not just an illusion. If an electrode is slowly advanced through somatosensory cortex along a path perpendicular to the cortical surface, all the cells encountered respond with about the same latency to the same type of stimulus delivered to about the same region of the body. Similarly, all the cells along a vertical path through visual cortex respond best to bars or edges with the same orientation in about the same part of the visual field (see Fig. 17-35A); if the electrode is moved 50 μm or so across the surface of the cortex, cells with a different preferred stimulus orientation are encountered. Furthermore, most cells along such a vertical path respond better to stimulation of one eye than to stimulation of the other eye; cells in a nearby vertical region may have not only a different preferred stimulus orientation but also a different preferred eye. The picture that has emerged is one in which the cortex is organized into vertical slabs or columns, each 50 to 500 μm wide, in which some parameter (e.g., stimulus orientation) is constant for all cells. Vertical slabs of different types (e.g., stimulus orientation or ocular dominance) intersect one another in patterns that are still not fully understood.
This kind of columnar organization probably reflects a general strategy used in the construction of neocortex. For most areas this is currently impossible to test physiologically because we cannot define the “best” stimulus for the cells in most parts of the cortex. However, some anatomical tracing techniques make it possible to visualize the columns (see Fig. 17-35C), and there are indications that columnar organization is widespread. For example, in at least some cortical areas, afferents from the thalamus, from other ipsilateral cortical areas, and from contralateral cortical areas end in vertical slabs separated by slabs that do not receive that particular kind of input. Throughout the neocortex, the basic building blocks appear to be “minicolumns” about 50 μm in diameter containing about 100 neurons; dozens of minicolumns, linked by horizontal intracortical connections, make up larger functional modules such as the hypercolumns in visual cortex (see Fig. 17-35A).
Neocortical Areas Are Specialized for Different Functions
Different Neocortical Areas Have Subtly Different Structures
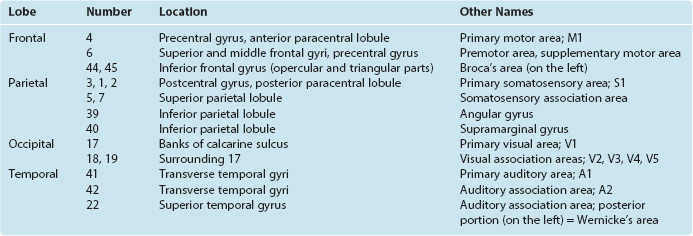
Seeing that various cortical areas are structurally distinct from one another in fairly obvious ways (e.g., granular versus agranular cortex [Fig. 22-6A] or striate versus extrastriate cortex [see Fig. 17-31]), a number of anatomists have sought to map the cortex in terms of these differences and often of considerably more subtle differences. One mapping system whose terminology has come into widespread use is that devised by Korbinian Brodmann, who divided the neocortex of each hemisphere into 44 areas (Fig. 22-10). The boundaries between many of these areas are not precise, as they often grade into each other by degrees. In addition, as noted previously, the correlation of functions with specific anatomical areas is not nearly as precise as was once hoped. Nevertheless, many of the areas described by Brodmann correspond remarkably well to areas defined by other measures of connection or function, and many of the numbers proposed by him are commonly used for reference purposes (Table 22-1).
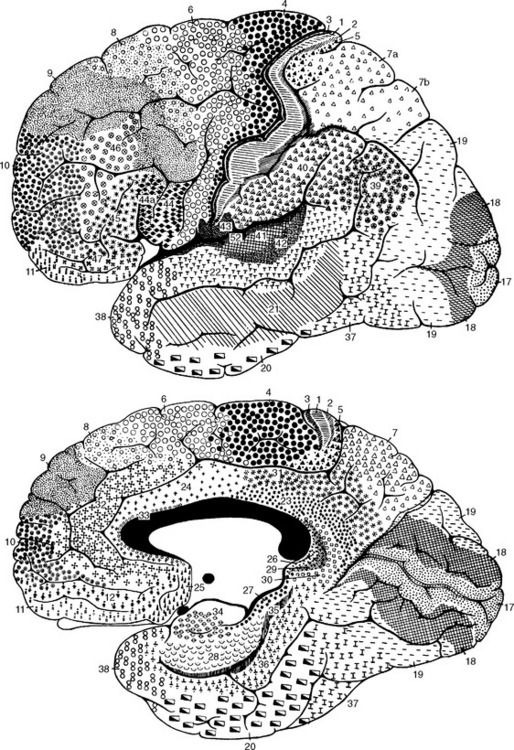
Figure 22-10 Brodmann’s anatomically defined areas of the human cerebral cortex (see Table 22-1).
(From Brodmann K: Vergleichende Lokalisation lehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues, Leipzig, 1909, JA Barth.)
Although each of us has roughly the same total amount of cerebral cortex, there are surprisingly large variations in the sizes of particular areas. The areas of visual, somatosensory, and motor cortex may vary by a factor of 2 to 3 among normal individuals. Because the total neocortical area is much more constant than this, someone with a larger than average visual cortex presumably has other areas that are smaller than average. Whether these differences in area are correlated with differences among individuals in various skills and functional capacities is not known. *
There Are Sensory, Motor, Association, and Limbic Areas
The neocortex of each cerebral hemisphere is usually considered to be made up of primary sensory areas (receiving inputs from thalamic sensory relay nuclei), a primary motor area (giving rise to much of the corticospinal tract), association areas, and limbic areas. Somatosensory cortex occupies the postcentral gyrus, visual cortex the banks of the calcarine sulcus, auditory cortex the transverse temporal gyri, and motor cortex part of the precentral gyrus. These areas are characterized by a topographical organization in which the body surface, the range of audible frequencies, or the outside world is mapped onto the cortical surface (see Figs. 3-30, 14-19, and 17-29). These maps are distorted (Box 22-1; Fig. 22-11), so that highly discriminating or finely controlled parts of the nervous system or body have disproportionately large representations (e.g., the fovea in visual cortex, the fingers in motor and somatosensory cortex).
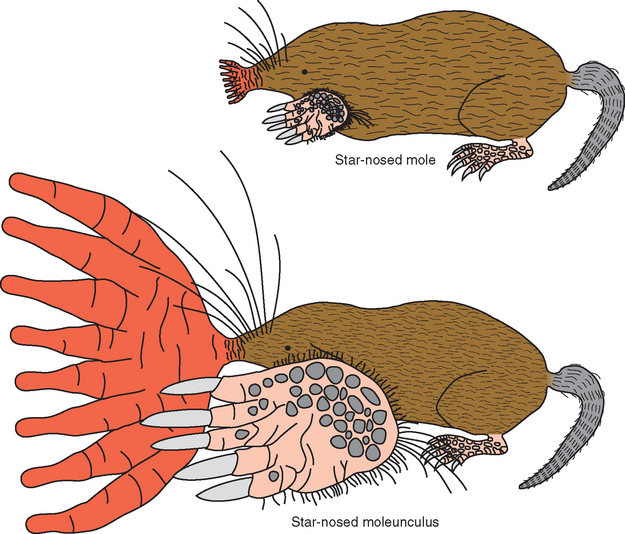
BOX 22-1 Star-Nosed Moles, Revisited
Star-nosed moles (see Box 9-1) have an elaborate array of 11 appendages, or rays, surrounding each nostril that are used for somatosensory exploration of their environment. Corresponding to the behavioral importance of these rays, more than 50% of the mole’s somatosensory cortex is used to process information from them (Fig. 22-11). Further details of the somatosensory map, though not apparent in Figure 22-11, also make functional sense. The moles move their noses around as they travel through their tunnels, contacting objects more or less at random with the rays. If contact is made by one of the first 10 rays, the animal reorients its nose so that the object can be explored in more detail by ray 11, a small, ventrally directed ray just above the mouth. If the object feels like it might be good to eat, it gets gobbled up. Interestingly, ray 11 has fewer Eimer’s organs (see Fig. 9-11) than almost any other ray, but each of these ray-11 organs has four times more cortical space devoted to it than do the organs from the other rays. This behavior pattern and cortical organization have been likened to the way we detect a visual target in the periphery and then point our foveae at it.
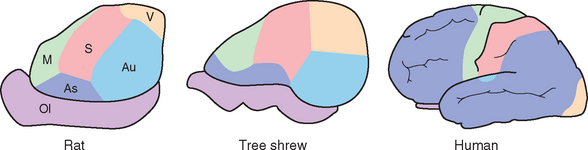
These primary areas are the cortical zones most directly related to the outside world, and they account for most of the cortex in many mammals. Primates, especially humans, have vast repertoires of ways to use and respond to sensory inputs; this is thought to result from a progressive increase in the amount of association cortex over the course of mammalian evolution (Fig. 22-12). Association cortex is commonly divided into two broad types. Some of the areas adjacent to a primary area are unimodal association areas (Fig. 22-13), devoted to an elaboration of the business of that primary area. Thus areas 18 and 19, which surround the primary visual cortex, are part of the visual association cortex. Similarly, parts of the superior parietal lobule, much of the superior temporal gyrus, and premotor and supplementary motor cortex are involved in somatosensory, auditory, and motor functions, respectively. This still leaves the inferior parietal lobule and large portions of the frontal and temporal lobes. Neurons in these areas typically respond to multiple sensory modalities and may change their response properties under different circumstances. For example, a neuron in the inferior parietal lobule might respond to a visual stimulus, but only if it is something interesting, such as a cue or a piece of food. These multimodal or heteromodal association areas are therefore thought to be concerned with high-level intellectual functions.
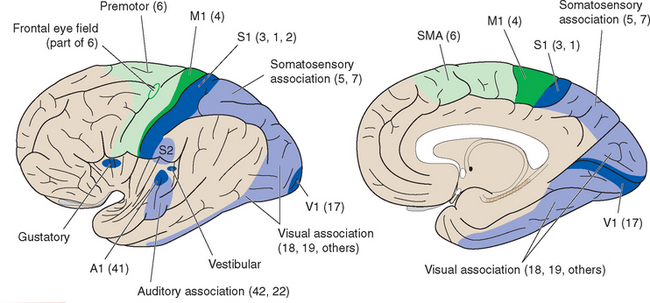
Figure 22-13 Summary diagram of primary and unimodal association areas. The lateral view, as in Figure 22-6, is drawn as though the lateral sulcus had been pried open, exposing the insula. Visual association cortex is particularly extensive in primate brains, occupying not only most of the occipital lobe but also much of the temporal lobe. Many of these various functional areas are associated with one or more of Brodmann’s anatomically defined areas, although sometimes the correspondence is only approximate; commonly used Brodmann numbers are indicated in parentheses (area 2 usually does not extend onto the medial surface of the hemisphere). A1, M1, S1, V1, primary auditory, motor, somatosensory, and visual cortex; S2, second somatosensory area; SMA, supplementary motor area.
(Modified from von Economo C: The cytoarchitectonics of the human cerebral cortex, Oxford, 1929, Oxford University Press.)
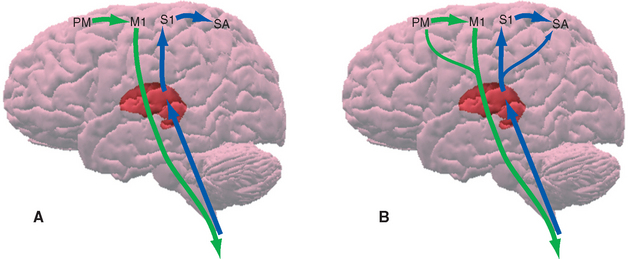
Although there is a great deal of validity to this broad view of cortical organization, it is also a considerable oversimplification. For one thing, the distinction between primary areas and association areas is not nearly as clear as the traditional formulation implies. One example given in an earlier chapter is the finding that the corticospinal tract originates not just from the classical primary motor cortex but also from other areas, including somatosensory cortex. As another example, there are types of cortex intermediate between primary and association (see Fig. 22-15), just as there are transitional areas between isocortex and allocortex. In addition, the concepts that primary sensory areas receive all the input (which is then acted on in a more complex fashion by the association cortex) and that motor activity is formulated in association areas and then funnels down to the primary motor area for expression are certainly not completely correct. Monkeys (and humans as well) with extensive damage to the precentral or postcentral gyrus are not rendered unable to move or to perceive tactile stimuli. They are impaired in these capacities, but the fact that they suffer only a partial disability indicates that other cortical areas also play a role and that these other areas can function independently of the primary areas, at least to some extent * (Fig. 22-14). The details of how different areas of the cortex and other parts of the CNS cooperate to produce a simple voluntary movement or a simple visual perception are still largely mysterious, but progressively more is becoming understood.
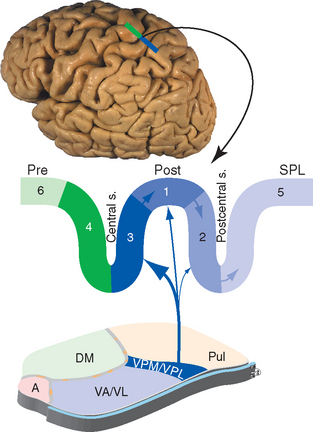
Figure 22-15 Pattern of connections of somatosensory cortex, as seen in a section through the precentral (Pre) and postcentral (Post) gyri and the superior parietal lobule (SPL). Numbers refer to Brodmann’s areas (see Fig. 22-10 and Table 22-1). Moving from area 3 to 1 to 2, each successive area receives less input from the thalamus and more from other somatosensory cortical areas. A, anterior nucleus; DM, dorsomedial nucleus; Pul, pulvinar; VA/VL, ventral anterior and ventral lateral nuclei; VPM/VPL, ventral posteromedial and ventral posterolateral nuclei.
Primary Somatosensory Cortex Is in the Parietal Lobe
Somatosensory information traveling rostrally in the medial lemniscus and the spinothalamic tract relays in the ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei and projects through the posterior limb of the internal capsule mainly to areas 3, 1, and 2. These are three long, parallel strips of cortex that together occupy almost the entire postcentral gyrus; most of area 3 is in the posterior wall of the central sulcus, and most of area 2 is in the anterior wall of the postcentral sulcus. These areas not only are structurally distinct from one another, but they also differ in their connections and properties; the body surface is mapped separately in each area, but when going from area 3 to 1 to 2, the characteristics change from primary somatosensory cortex to the beginnings of somatosensory association cortex (Fig. 22-15). Cells in area 3 receive most of the thalamocortical projections from VPL and VPM; cells in areas 1 and 2 receive progressively less input from the thalamus and progressively more from other cortical areas. Cells in area 3 have receptive fields that reflect activity in particular kinds of receptors, whereas those in areas 1 and 2 have more complex receptive fields that respond to things such as limb position or the shape of an object touching the skin. In the strict sense of the term, area 3 is primary somatosensory cortex, but because the postcentral gyrus as a whole is the most prominent area concerned with somatic sensation, it is usually referred to as the primary (first) somatosensory area (or S1).
Stimulation of the postcentral gyrus in conscious humans produces sensations usually described as tingling or numbness in a contralateral part of the body whose location is related in an orderly way to the site stimulated (see Fig. 3-30). The sensations generally do not resemble those caused by natural stimuli such as bending a hair or touching the skin, presumably because electrical stimulation of the cortex is a poor imitation of the pattern of activity set up by natural stimuli. Interestingly, sensations of pain can rarely be elicited from the postcentral gyrus, and the way pain is represented in the cerebral cortex continues to be something of a mystery. Large lesions affecting the entire postcentral gyrus cause considerable impairment of the finer aspects of somatic sensation (such as judging the exact location or intensity of a stimulus) and a serious deficit in the sense of position and movement of the affected parts, but such damage does not abolish tactile sensation or the sensation of pain. Indeed, on the few occasions when removal of the postcentral cortex was tried as a treatment for intractable pain, the patient’s pain was usually relieved only partially and briefly, and this was often followed by a hyperpathic state reminiscent of thalamic pain. In contrast, small lesions affecting only the part of the postcentral gyrus adjacent to the central sulcus cause not a loss of pain and temperature sensation but rather difficulty localizing painful stimuli in the somatotopically appropriate part of the body on the contralateral side. Corresponding to this clinical observation, neurons specifically responsive to painful stimuli have been found in the somatosensory cortex of monkeys at about the junction between areas 3 and 1. However, processing of pain information is not the exclusive province of S1. Pain-sensitive neurons have also been found in S2 and other areas, and functional imaging studies have demonstrated increased blood flow in S1, S2, part of the insula, and anterior cingulate cortex in response to painful stimuli. S1 is now thought to be important for localizing painful stimuli, and the other areas (especially the insula and cingulate gyrus) are thought to be responsible for the unpleasantness of pain; the reasons why large cortical lesions cause a hyperpathic state are not yet clear.
Primary Visual Cortex Is in the Occipital Lobe
The retinotopic projection from the lateral geniculate nucleus to the banks of the calcarine sulcus, conveying information about the contralateral visual field, is described in Chapter 17. This primary visual cortex (V1, also called striate cortex; see Fig. 17-31) corresponds to area 17 of Brodmann’s map. Peripheral parts of the visual field are represented anteriorly, the fovea has a disproportionately large representation located posteriorly, and the vertical meridian is represented along the upper and lower borders of area 17 (see Fig. 17-29). Although area 17 looks fairly small on maps such as those in Figures 22-10 and 22-13, it really occupies a substantial amount of the cortical surface and appears small only because most of it forms the walls of the deep calcarine sulcus.
A two-part visual association cortex occupies the rest of the occipital lobe. Area 18 surrounds area 17 and is itself surrounded by area 19. This association cortex receives its visual information both from area 17 and via the superior colliculus–pulvinar pathway. Areas 18 and 19 are themselves complex mosaics of smaller, retinotopically organized areas—one interested in the movements of objects, another in the colors of objects, and still others in other properties. Additional visual association areas occupy much of the temporal lobe (Fig. 22-13), reflecting the importance of vision for primates.
The relative roles of primary visual cortex and visual association cortex in human vision are not completely understood. However, it is safe to say that the primary area is extremely important, because its destruction results in a total or near-total loss of conscious awareness of visual stimuli. A simplified view of its function, then, is that the primary visual cortex does some initial processing of inputs from the lateral geniculate nucleus (e.g., combining inputs from the two eyes and beginning to analyze depth), then distributes this information to the various subareas of visual association cortex where motion, color, and other parameters are analyzed more elaborately (see Fig. 17-36). If this is true, bilateral lesions of visual association cortex could conceivably disrupt single aspects of visual function. (Such cases would also be extremely rare, because they would have to involve symmetrically placed areas and spare the optic radiations.) A few cases have in fact been reported (see Box 17-2) in which bilateral damage to the inferior surfaces of the occipital lobes caused color blindness or in which more lateral damage near the occipital-temporal junction caused motion blindness.
Primary Auditory Cortex Is in the Temporal Lobe
The superior surface of the temporal lobe forms one wall of the lateral sulcus. One or two transverse temporal gyri (of Heschl; see Fig. 14-18) cross the posterior part of this surface and form Brodmann’s areas 41 and 42. Area 41 is granular cortex (like areas 3 and 17) and receives most of the auditory radiation from the medial geniculate nucleus via the sublenticular part of the internal capsule; thus it serves as primary auditory cortex, or A1 (Fig. 22-16). Just as the body is mapped onto the postcentral gyrus (somatotopy) and the retina is mapped onto striate cortex (retinotopy), the spectrum of audible frequencies is mapped onto area 41 (tonotopy; see Fig. 14-19). Area 42 is adjacent to area 41 and receives auditory information from both area 41 and the medial geniculate nucleus. This is analogous to the arrangement found in the second somatosensory area (S2), so area 42 is often referred to as A2. The cortex surrounding area 41 in monkeys includes at least four different subareas, each with its own tonotopic map. The same is assumed to be true for humans, but the exact details of the arrangement of these multiple maps with respect to area 42 are not known. Area 42 is itself flanked by area 22, which forms much of the superior temporal gyrus and is called the auditory association cortex.
At levels rostral to the cochlear nuclei, both ears are represented in the auditory pathway of each side of the brain, although the contralateral ear predominates (see Chapter 14). As a result, even total destruction of auditory cortex on one side has relatively little effect. An individual with such damage may have some difficulty localizing sounds on the contralateral side and may have some subtle hearing loss that is greater for the contralateral ear, but the deficits are not nearly comparable in magnitude to those that follow unilateral damage to somatosensory or visual cortex. If the auditory association cortex of area 22 is damaged in the dominant hemisphere, severe language problems ensue, as discussed later in this chapter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree