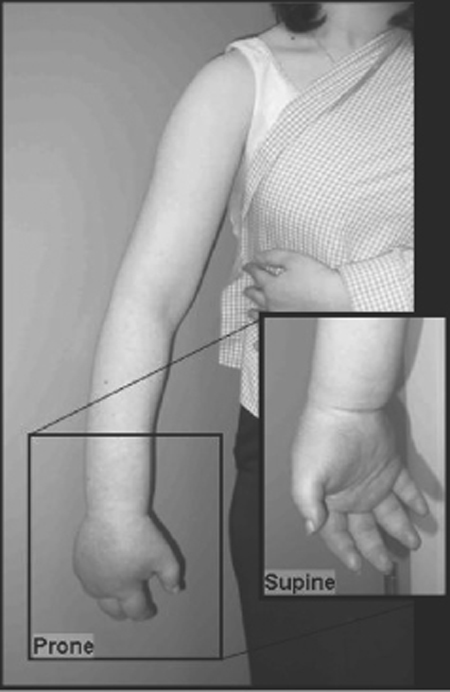
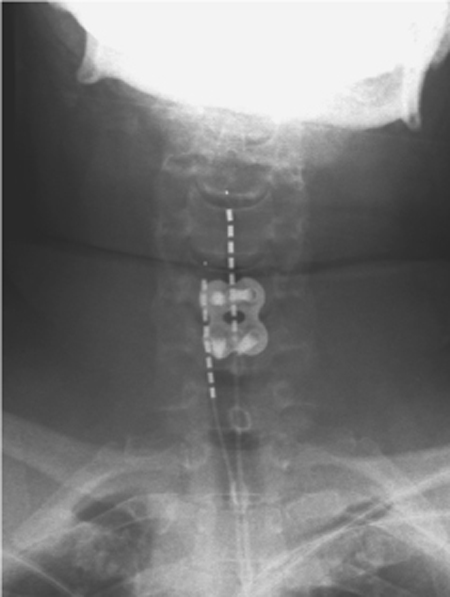
55 Complex Regional Pain Syndrome A 53-year-old female was using a disposable camera when she got a minor burn or shock from the flash. Immediately thereafter, she developed shooting pain that she described as “deep and aching. ” The pain became constant in nature, graded a 5–6/10 in severity, and the arm and hand became “swollen and purple” (Fig. 55–1). The pain worsened over time, and she rapidly became unwilling to use the arm due to severe pain on movement. She was treated initially with short-acting opiates by her primary care physician. After referral to a neurologist, she was treated with anticonvulsant and tricyclic antidepressant medications with little response. She was sent for a cervical spine magnetic resonance imaging (MRI) scan that revealed a C5–6 central disk bulge. Physical therapy was too painful, and the patient discontinued it. A stellate ganglion block helped temporarily, lasting only a few hours. Meanwhile, after referral to a neurosurgeon she underwent a C5–6 anterior cervical diskectomy and fusion that did not offer any relief. She was subsequently diagnosed with complex regional pain syndrome (CRPS), sent for psychological evaluation, and restarted on a physical therapy program. Although she initially experienced some slight improvement in her pain levels, she soon plateaued in her recovery. At this point she underwent cervical spinal cord stimulation to obtain pain relief in her right arm, and intraspinal peripheral nerve root stimulation to obtain pain relief in her hand (Fig. 55–2). Three months following treatment, with a combination of anticonvulsant medication, physical therapy, psychological counseling, and stimulation, she has regained full use of the right arm. Complex regional pain syndrome Figure 55–1 A 53-year-old female with complex regional pain syndrome. Right hand shows severe edema and vasomotor changes. CRPS is a poorly understood, chronic pain disorder that occurs following nerve injuries. Typically, patients experience horrific pain that far exceeds that expected from the mechanism of injury. In 1864 the diagnosis of “causalgia” was introduced by Weir Mitchell. Early reports described soldiers returning from the battlefield with persistent, severe, burning pain after sustaining gunshot wounds to the extremities with consequent peripheral nerve injuries. Causalgia was the syndrome of severe burning pain sometimes seen after these injuries. Later descriptions of the syndrome reported edema, atrophy, and trophic changes in the affected extremity. Early descriptions suggested that peripheral fiber irritation initiated cycles of perpetual neuronal firing in the dorsal horns, activating ascending central pain pathways. The term reflex sympathetic dystrophy was proposed in 1946, implicating the sympathetic nervous system as a perpetuator of the symptomatology of the disorder. Support for the concept of “sympathetically maintained pain” was initially based upon improvement in patients following sympathetic nervous system local anesthetic blockade. Unfortunately, initial enthusiasm for sympathetic blockade as both a diagnostic as well as a therapeutic procedure waned because many cases seemed not to involve the sympathetic nervous system. Despite this, these blocks are still widely used in many pain management centers. Others proposed that neurogenic inflammation in the acute stages of CRPS could result in the temperature changes, edema, and trophic changes seen in this disorder. In addition, neuropeptides can be released peripherally and centrally, causing hyperhidrosis and modulation of primary afferents, respectively. More recently, studies have shown an increase of substance P receptors, elevation of soluble tumor necrosis factor (TNF) receptors, and an enhancement of TNF-α activity in patients with allodynia (pain resulting from normally nonpainful stimuli). Finally, autoantibodies may be involved in patients with CRPS. Thus data are still emerging to better understand the physiology and anatomy of CRPS. Figure 55–2 Anteroposterior x-ray of the cervical spine, showing dorsal column and intraspinal peripheral nerve root stimulator electrodes. Recent functional imaging studies have begun to clarify some of the relevant neuronal circuitry involved in CRPS. For example, CRPS patients exhibit a reorganization of the primary somatosensory cortex as seen on magnetoencephalography. This may in part explain why patients experience symptoms within adjacent or even distant body parts, even though the inciting injury was quite localized. This cortical reorganization has been shown to reverse as patients undergo effective CRPS treatment. Functional imaging studies in CRPS have revealed characteristic areas of activation following stimulus-induced hyperalgesia. Stimulation led to increased activation of the contralateral primary somatosensory, bilateral secondary, bilateral insula, contralateral associative-somatosensory, bilateral frontal, and bilateral anterior cingulate cortices. Taken together, these studies suggest that discrete cortical changes occur in patients with CRPS, can be detected on functional imaging, and vary in response to treatment. A new classification system was outlined in an effort to clarify the diagnostic criteria for the different types of these disorders, renamed complex regional pain syndrome. One important recognition by the renaming body was the lack of mechanistic knowledge of these disorders, instead relying on more descriptive nomenclature. Hence, the new naming scheme avoids speculative, mechanistic labels, such as sympathetic or reflex, and instead uses generally descriptive terms such as regional and complex.
 Case Presentation
Case Presentation
 Diagnosis
Diagnosis
 Anatomy
Anatomy
 Characteristic Clinical Presentation
Characteristic Clinical Presentation
| 1. | Antecedent noxious event that either does not cause (CRPS-1) or causes (CRPS-2) a discrete nerve injury |
| 2. | Ongoing spontaneous pain and mechanical hyperalgesia that is disproportionately greater than expected from the initial injury and that exceeds the confines of a single nerve or nerve root distribution |
| 3. | Presence of objective findings, at some point in time, such as temperature changes, sudomotor abnormality, edema, or vasomotor abnormality |
| 4. | Absence of another explanatory diagnosis |
The new classification scheme was proposed as follows (Table 55–1). CRPS type 1 generally represents the clinical spectrum previously labeled as reflex sympathetic dystrophy. It occurs following a noxious event that does not involve a discrete peripheral nerve injury. Symptoms must consist of spontaneous or evoked pain that exceeds the confines of a single nerve distribution, disproportionate to the injury caused by the initial traumatic event. Signs must consist of some other abnormality in the affected body part, such as temperature changes, edema, color changes, or deranged sudomotor activity. CRPS type 2 generally represents the clinical spectrum previously labeled as causalgia. It consists of the same criteria as CRPS-1, except that a discrete nerve injury must have occurred. In both cases, the patient’s symptomatology must not be the result of another pathophysiological process.
Pain, the most important finding in patients with CRPS, typically consists of mechanical hyperalgesia or allodynia or both. Many patients describe having pain at rest associated with an aching and burning sensation deep in the affected extremity. Such severe pain may lead to kinesiophobia, in which the patient is extremely unwilling to move the extremity or even have it touched. Vasomotor and temperature changes may be prominent in the affected body part as well. Patients with CRPS often have temperature differences between the affected and nonaffected limbs, and this distinctive feature can help distinguish CRPS from other pain syndromes. It has been well documented that the symptoms and signs of CRPS may begin in one body part and later spread to another. Alternatively, CRPS may begin simultaneously in bilateral body parts or appear in one part, disappear, and reappear in the same part months to years later. Trophic changes of the skin, hair, and nails may occur, followed by joint stiffness and contractures in severe cases. For example, after the initial onset of symptoms of CRPS, there is an increase in hair and nail growth. Over time, however, hair and nail growth becomes reduced and there is associated atrophy of the skin. Patients may have other findings as well, such as weakness or tremor, but these are not included as part of the diagnostic criteria. Motor disturbances include weakness that can acutely be due to decreased range of motion secondary to edema, or chronically due to contraction and fibrosis. CRPS may occur in patients of nearly any age and has been well documented in adults as well as young children. CRPS patients often have coexisting psychological morbidity; however, it is unclear whether these are causally related or merely epiphenomena. Medical practitioners are often unfamiliar with these psychological issues, as well as of CRPS itself. Thus many patients are met with suspicion and not taken seriously.
 Differential Diagnosis
Differential Diagnosis
Any given symptom or sign seen in CRPS may be found in other clinical disorders as well. It is important to emphasize that CRPS is often a diagnosis of exclusion, and other etiologies for shared clinical findings must be explored. For example, peripheral edema may be present in deep vein thrombosis, fractures, sprains, and thrombophlebitis. Similar patterns of pain can be seen in peripheral neuralgias, erythromelalgia, and diabetic peripheral neuropathy. Distinguishing CRPS from diabetic peripheral neuropathy may be especially problematic because both may involve severe neuropathic pain, edema, and trophic changes that have spread beyond the confines of a single peripheral nerve or nerve root distribution. A history of poorly controlled diabetes and characteristic changes on electrodiagnostic studies help in many cases to differentiate diabetic neuropathy from CRPS.
The terms sympathetically maintained pain (SMP) and sympathetically independent pain (SIP) have been (incorrectly) used interchangeably with CRPS. The former terminology implies that a patient benefited from interruption of the sympathetic activity with either pharmacological or nerve block methods. The latter implies that a patient did not. The misuse of the terms SMP and SIP have obfuscated their relationship with CRPS. SMP and SIP are generally descriptive terms that do not necessarily represent independent clinical entities, any more than the terms allodynia or hyperalgesia do. CRPS, on the other hand, represents a clinical diagnosis and includes patients that respond to sympathetic blocks, as well as ones that don’t.
 Diagnostic Tests
Diagnostic Tests
CRPS is predominantly a clinical diagnosis but can be supported by certain diagnostic examinations. Unfortunately, a single, reliable, sensitive, and specific diagnostic test for CRPS is not available. It is important to emphasize that a negative result with any of following tests cannot exclude the clinical diagnosis of CRPS. Quantitative sensory testing and quantitative sudomotor axon reflex testing showed initial promise as diagnostic tools for sensory and sweating abnormalities associated with CRPS. Unfortunately, they have not proven to be effective in diagnosing CRPS. Radiography can show spotty osteoporotic changes in affected areas, but this occurs in less than half of CRPS cases. Three-phase bone scanning can occasionally show increased bone metabolism in CRPS patients, but several other clinical conditions can yield the same findings. MRI can be used to exclude other pathologies, which is a clinical criterion in the diagnosis of CRPS. A cold pressor test performed with thermographic imaging test can observe vasoconstrictor response. Laser Doppler flow studies have been utilized to evaluate background vasomotor control. Other studies include blood-flow studies, thermography, cutaneous temperature measures, sympathogalvanic reflexes, and volumetric displacement changes. Unfortunately, all the aforementioned studies have either or both poor sensitivities or specificities and cannot be used solely to diagnose CRPS
 Management Options
Management Options
Though CRPS has been the focus of a substantial amount of research in the past several years, there is only a rudimentary understanding of the pathophysiology of this disease. This lack of understanding has crippled the best efforts to design highly effective treatment strategies. Historically, many types of treatments have been proposed for CRPS. Limb amputation has been described as a treatment for CRPS. Unfortunately, pain relief occurred in only ˜6% of amputated limbs. Ablative, and often drastic, treatments such as this have largely been abandoned.
Treatment of CRPS consists of a multidisciplinary approach whose goal is to improve patient function, not to attempt a “cure. ” Physical therapy targeted at the affected body part is the mainstay of treatment. Up to 92% of children with CRPS became symptom-free when they adhered to a consistent physical therapy program. Analgesics, beginning with the antidepressant and anticonvulsant medications, and then opioids if necessary, are used to provide acceptable levels of analgesia so that physiotherapy may be undertaken. Steroids may be beneficial if utilized early in the course of the disease. Psychological evaluation, and ongoing counseling as needed, may be required for the patient to enjoy continued progress with therapy. Goals of psychological intervention are to identify and address potent psychological amplifiers of pain, such as stress, depression, poor coping strategies, and unresolved conflicts. Regional anesthetic techniques, such as regional blocks or sympathetic blocks, may offer sufficient, albeit temporary, pain relief to permit physiotherapy to progress. Sympathetic blocks have played an important role in the treatment of CRPS, especially given that the early symptoms respond so well to it. These blocks have evolved over time and now include intravenous regional blocks, stellate and lumbar sympathetic blocks, paravertebral sympathetic blocks, and epidural blocks. A variety of anesthetic block therapies have been evaluated, such as clonidine, phentolamine, phenylephrine, reserpine, guanethidine, droperidol, and ketanserin. To date, the best randomized, active-controlled, crossover studies that investigated sympathetic blockade have shown that the combination of lidocaine and bretylium results in significant pain reduction. Whatever the drug of choice may be, sympathetic blockade is indicated when medical pharmacotherapy fails to provide appropriate pain alleviation for the patient to reestablish a physical therapy program. Ketamine infusions have also been utilized in the treatment of CRPS. One study reported that a low-dose infusion of ketamine was a successful treatment option for patients with intractable CRPS, offering relief of pain in 75% of study subjects. Finally, the relatively new technique of repetitive transcranial magnetic stimulation of the motor cortex has been shown to provide some relief in patients. Cases refractory to these nonoperative measures should proceed with a trial of neuromodulation.
 Surgical Treatment
Surgical Treatment
Neuromodulation is the process by which the relevant pain pathways are influenced by electrical or pharmacological means, generally with the use of an implantable device. In most cases these techniques are reversible, nonablative, and noncurative. Though the mechanisms of neuromodulation often remain obscure, they likely achieve symptomatic relief through some combination of direct inhibition of relevant neural circuitry as well as activation of inhibitory circuitry. Peripheral nerve stimulation involves placement of an electrode directly on a peripheral nerve, relieving pain within the distribution of that nerve. This modality has been reported to be effective in the treatment of CRPS, with over 60% enjoying long-term pain relief. Intrathecal baclofen has been reported to significantly improve the dystonia associated with CRPS.
Spinal cord stimulation (SCS) was first reported in the 1980s as an effective treatment for CRPS. This therapy has been validated in more recent, prospective studies and has a roughly 56% initial efficacy rate, maintaining a 57% success rate by 2 years. Unfortunately, success rates lost statistical significance after ˜3 years compared with a control group. Though this clinical trial convincingly demonstrated the short-term advantages of spinal cord stimulation over less invasive measures in selected patients, overall only about half of the stimulator patients obtained “much improvement.” Studies of the cost effectiveness of spinal cord stimulators combined with physical therapy show SCS can save approximately $60,000 over a lifetime compared with physical therapy alone. Novel techniques of neurostimulation, such as intraspinal nerve root stimulation, transforaminal peripheral nerve root stimulation, and motor cortex stimulation, and newer stimulation technologies such as rechargeable systems and current steering, have been recently developed. These methods now provide the pain practitioner greater flexibility and control in choosing a neurostimulator system that is more ideally suited for individual patients based upon their pattern and severity of pain.
In treating patients with CRPS, we initially employ the treatment measures already outlined. Patients undergo a course of aggressive treatment with anticonvulsant and/or antidepressant medications. Sometimes other medication classes, such as the opiates, are employed as well. Noninvasive pain management techniques, such as transcutaneous electro-nerve stimulator (TENS) units, acupuncture, or Lidoderm patches (Endo Pharmaceuticals, Chadds Ford, PA), are sometimes utilized. Once the pain is under at least modest control, the patient then undergoes physical therapy and psychological support. If after 4 to 6 weeks of this multimodality approach the patient either fails to progress or initial progression plateaus, then we consider the patient for a trial of neurostimulation.
Patients that have pain localized to a particular region, such as one or more extremities, are typically better candidates than patients that have diffuse pain involving large regions of the body. Pain in the lower extremities is typically treated with a thoracic spinal cord stimulator. Pain in the upper extremities is typically treated with a cervical spinal cord stimulator. Trial electrodes are generally placed in a percutaneous fashion, whereas the permanent electrodes are typically dual-channel, paddle leads placed under local anesthetic through a small hemilaminotomy on the midline. Patients with extensive degenerative spinal arthritis or who are at high risk for perioperative complications secondary to medical comorbidities undergo placement of permanent percutaneous leads rather than laminectomy leads to shorten operative time, surgical dissection, and postoperative pain. After a period of trial stimulation, which typically lasts 7 days, the electrodes are removed and replaced with permanent versions connected to an implantable pulse generator. The decision on whether to utilize a rechargeable implantable pulse generator depends in part upon the patient’s electrical requirements during the trial period. Patients with high stimulation frequencies, intensities, or complex patterns of stimulation are typically offered rechargeable implant-able pulse generators. Patients with very low electrical requirements or patients with diminished capacity, such as older patients with short-term memory deficits, may be better treated with a nonrechargeable generator because these systems are logistically much simpler for patients to manage on a day-to-day basis.
Spinal cord stimulation typically treats the majority of patients satisfactorily. Some distributions of pain, however, are poorly or inconsistently treated with SCS. For example, pain along the bottom of the foot is better treated with a transforaminal S1 stimulator electrode. This technique is similar to SCS, except that the electrode is placed in a retrograde fashion (cephalocaudal), and the electrode is steered out into the neural foramen of S1. In this manner, stimulation paresthesias may be generated selectively in the distribution of the target nerve. Pain on top of the foot, similarly, is best treated with a transforaminal L5 stimulator. Pain across the knee may be treated with a transforaminal L3 stimulator. Intraspinal nerve root stimulation is similar to SCS, except that the electrode is placed laterally in the spinal canal to target the spinal nerve dorsal rootlets before they exit the canal. Groin pain is effectively treated with an intraspinal nerve root electrode placed in the gutter along the T12, L1, and L2 nerve rootlets on the affected side. Pain in the hand can be treated adequately with an intraspinal nerve root electrode along the C6, C7, and C8 rootlets on the affected side, as in Fig. 55–2.
Typically, the surgical procedures are performed with the patient under conscious sedation on an outpatient basis. Complications are few, and most occur as a result of hardware complications such as lead migration, lead fracture, exhaustion of battery life, or the need to revise an electrode position because the pattern of the patient’s pain has changed. Infection occurs at a rate of roughly 1% per year, slightly higher in diabetic patients, patients on chronic steroids, and the elderly.
With increased knowledge of the pathophysiology of CRPS and the mechanisms of action of neurostimulation, it is hoped that patient selection and treatment outcomes from these treatment options can be enhanced. Perhaps novel, less invasive, treatment approaches could be designed as well.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


