Chapter 6 Cortical and Subcortical Brain Mapping
The first goal of brain surgery, especially in neuro-oncology, is to optimize the extent of resection (EOR) of the lesion. Indeed, maximal resection of glioma, when possible, is currently the first treatment to consider, both in low-grade gliomas (LGGs)1 and in high-grade gliomas.2 In the recent series measuring objectively the EOR on repeated postoperative MRI, all of them supported EOR as a statistically significant predictor of overall survival. In WHO grade II gliomas, when no signal abnormality was visible on control MRI, especially on FLAIR-weighted imaging (i.e., the so-called “complete resection”), patients had a significantly longer overall survival compared with patients having any residual abnormality. Interestingly, even in cases of incomplete tumor removal, patients with a greater percentage of resection had a significantly longer overall survival. In addition to the percentage of resection, the postoperative tumor volume is also a predictor of survival, with a significantly longer overall survival when the residue is less than 10 ml (“subtotal resection”) compared with more than 10 ml (“partial resection”).3 In glioblastomas, it was also shown that the complete removal of the enhanced part of the tumor controlled on postsurgical MRI increased the median survival around 17 months, instead of only 12 months if a residual enhancement was left.2
Consequently, to optimize the benefit-to-risk ratio of surgery, an increasing number of authors used functional mapping methods over the last decade. Indeed, considerable interindividual anatomofunctional variability was demonstrated in healthy volunteers.5 Furthermore, this variability is increased in cases of gliomas, due to cerebral plasticity, explaining why many patients have no or only a mild deficit before surgery, especially in slow-growing tumor such as LGG.6 It is thus mandatory, for every patient, to study the cortical functional organization, effective connectivity, and brain plastic potential, in order to tailor the resection according not only to oncologic but also to cortico–subcortical functional boundaries.
Presurgical Functional Brain Mapping: Advances and Pitfalls
Preoperative Neurocognitive Assessment
Gliomas, especially LGG, are usually revealed by inaugural seizures in young patients who have had a normal life, with no or only a mild neurologic deficit. However, recent extensive neuropsychological examinations have demonstrated that most of patients had cognitive disturbances, especially concerning working memory and executive functions.7 This is the reason why a systematic preoperative neurocognitive assessment is now recommended to search the possible neuropsychological deficit not identified by a standard neurologic examination, to adapt the surgical methodology (e.g., functional mapping under local anesthesia) to the results of this assessment, to benefit from a presurgical baseline allowing a comparison with the postsurgical evaluation, and to plan specific functional rehabilitation.
It is nonetheless puzzling to note that these deficits are not more pronounced, despite the frequent location of LGG in the so-called “eloquent areas.” This can be explained by mechanisms of brain reshaping allowing functional compensation in cases of slow-growing lesions. Indeed, it was shown that cerebral remapping was possible, with a recruitment of perilesional or remote areas within the ipsilesional hemisphere and/or recruitment of contra-hemispheric homologous areas. The recent integration of these concepts into the therapeutic strategy has resulted in dramatic changes in the surgical management of LGG patients, with an increase of surgical indications in eloquent regions classically considered “inoperable”.6
Preoperative Functional Neuroimaging: A Necessary Baseline
However, it is worth noting that FNI methods are not yet reliable enough at the individual scale, despite constant improvement efforts, mainly because the results depend on biomathematical models used for reconstruction. Regarding fMRI, correlations with intraoperative electrophysiology demonstrated that the sensitivity of fMRI was currently only around 71% for movement, and from 59% to 100% for language (specificity from 0% to 97%).8 Such discrepancies can be explained by a neurovascular decoupling in cases of glioma (blood-oxygen–level dependence response in the vicinity of gliomas does not reflect the neuronal signal as accurately as it does in healthy tissue), by inadequate tasks (not adapted to the location of the glioma and/or to the neurologic status of the patient), or to methodological problems (e.g., selection of the threshold). As a consequence, there is a risk of false negative and then to operate a patient without intraoperative mapping, although the glioma is actually located in crucial areas for the function, but not detected by preoperative FNI. Moreover, an erroneous interpretation of brain reshaping (“pseudoreorganization”) can be made. Finally, these methods are not able to differentiate the structures essential for the function, which should be surgically preserved, from those which can be functionally compensated and so potentially resected without permanent deficit. Thus, there is a double risk: first, failure to select a patient for surgery while the tumor was operable, and second, to stop the resection prematurely with a lower impact on the natural history of the glioma (or both).
The recent development of the diffusion tensor imaging (DTI) has also allowed the identification of the main bundles and their location in relation to the tumor. However, this new method needs to be validated before it can be used routinely for surgical planning, especially due to the fact that results of DTI, as FNI, strongly depend on the biomathematical models used for the fiber tracking. Indeed, comparison of distinct fiber-tracking software tools found different results, showing that neurosurgeons have to be cautious about applying tractography results intraoperatively, especially when dealing with an abnormal or distorted fiber tract anatomy. Furthermore, correlations between DTI and intrasurgical subcortical stimulation demonstrated that, despite good correspondence, DTI is not yet optimal for mapping language tracts in patients. Negative tractography does not rule out persistence of a fiber tract, especially when invaded by a glioma.9 Moreover, DTI enables study of the sole anatomy of the subcortical pathways, but not their function.
Intrasurgical Functional Brain Mapping: Toward a Hodotopic View of Brain Processing
Intraoperatively, the integration of multimodal imaging into frameless stereotactic surgery was extensively used in the past decade and referred to as “functional neuronavigation.” However, a randomized trial failed to demonstrate significant impact of navigation on postoperative results.10 It can be explained by the limitations of the presurgical neuroimaging detailed above, as well as to the high risk of intraoperative brain shift, due to surgical retraction, mass effect, gravity, extent of resection (especially for voluminous tumors), and cerebrospinal fluid leakage. Several technical improvements have been proposed to reduce the effects of this shift, but their reliability has still to be optimized: combination with intraoperative ultrasound, producing real-time imaging; use of mathematical models based on data from ultrasonography or digital images that track cortical displacement; and intraoperative MRI. Nevertheless, their actual value on the improvement of EOR and preservation of quality of life remains to demonstrate. As a consequence, invasive electrophysiologic investigations currently remain the “gold standard” when operating in eloquent brain structures.
First, the technique of somatosensory- and motor-evoked potentials was extensively used in the past decades for intraoperative identification of the central region. However, its reliability regarding the localization of the rolandic sulcus is not optimal, with accurate localization of the central sulcus reported only 91% to 94%. Estimation of the overall sensitivity and negative predictive value of this method is around 79% and 96%, respectively. Moreover, phase reversal recording identifies only the central sulcus itself, but offers no direct information on the particular distribution of motor function on the adjacent exposed cerebral structures. Also, whereas the method of motor evoked potentials was improved, when recording compound muscle action potentials, only the monitored muscles can be controlled, that is, there is an inability to detect and possibly avoid motor deficits in nonmonitored muscles. Next, monitoring of muscle action potentials does not mean monitoring of complex movements and action adapted to the environment, which is nonetheless the ultimate goal for the patient. Above all, intraoperative-evoked potentials cannot currently be used to map language, memory or other higher functions crucial for patients’ quality of life (for a review, see ref.11).
Intrasurgical Cortical and Subcortical Electrostimulation Mapping Methods
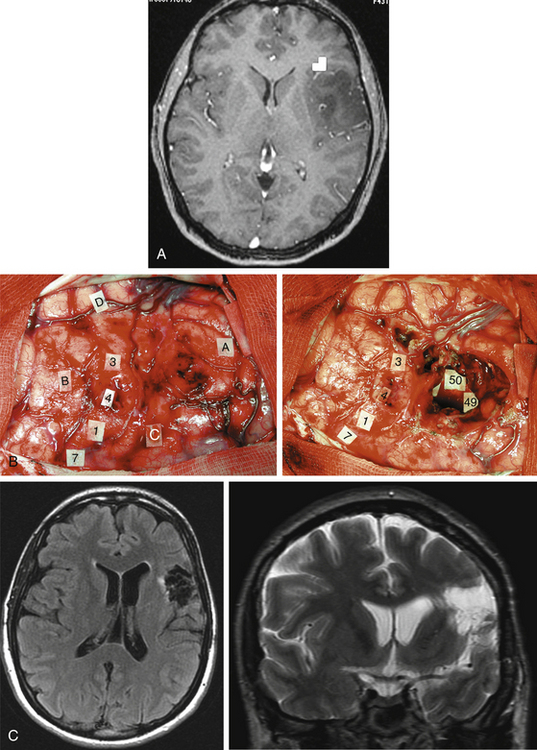
Taking into account the advantages and the limits of these different mapping techniques, more and more neurosurgeons advocate the additional use of intrasurgical electrostimulation mapping (IESM), under general or local anesthesia during surgery in eloquent areas.12,13 Indeed, except for tumors located within the motor structures, the mapping is performed in awake patients. However, as previously mentioned, since movements and action are more complex than single muscle contractions, it is also currently proposed to map the motor function under local anesthesia with active participation of the patient.14 The principle is to use IESM as a focal and transitory virtual lesion to obtain an individual functional map both at cortical and subcortical levels, and to test if a structure involved by a lesion is still crucial for the function—which is, for instance, observed in 15% to 20% of LGG cases. Stimulation of an essential area generates a transient disruption of the task performed by the patient, and this area should be preserved. An individual cortical mapping is thus obtained before the resection, which can be tailored according to functional boundaries (Fig. 6-1). Practically, a bipolar electrode tip spaced 5 mm apart and delivering a biphasic current (pulse frequency 60 Hz, single-pulse phase duration 1 millisecond) is applied to the brain. The current intensity adapted to each patient is determined by progressively increasing the amplitude in 1-mA increments from a baseline of 2 mA until a functional response is elicited, with 6 mA as the upper limit under local anesthesia, and with 16 mA as the upper limit under general anesthesia—with the goal of avoiding the generation of seizures. The patient is never informed when the brain is stimulated. At least one picture presentation without stimulation must separate each stimulation, and no site is stimulated twice in succession to avoid seizures. Each cortical site (size 5 × 5 mm, due to the spatial resolution of the probe) of the entire cortex exposed by the bone flap is tested three times. Indeed, it is admitted nowadays that three trials are sufficient to ensure whether an area is crucial for language, by generating disturbances during its three stimulations, and with normalization of the function as soon as the stimulation is stopped. This limitation of trials and tasks is required by the timing of the surgical procedure, because the patient is awake and can be tired at the end of the resection.
Interestingly, recent series show that the surgical procedure can be simplified by avoiding the use of intraoperative electrocorticography despite an equivalent reliability of electrical mapping and without increasing the rate of seizures.12 However, in cases of stimulation-induced seizures, the use of cold Ringer’s lactate is recommended to abrogate the seizure activity. In addition, some authors emphasized the value of “negative mapping” (no identification of eloquent sites) in the setting of a tailored cortical exposure.13 Although such recommendation is acceptable for high-grade gliomas, since the surgical goal is mainly to remove the enhanced part of the tumor, a negative mapping can be dangerous in surgery of diffuse LGG, especially in nonexpert hands. Indeed, due to the fact that LGG is poorly delineated, the limit of the resection will be essentially guided according to functional criteria. Because negative mapping can be due to false negative for methodologic reasons, it does not guarantee the absence of eloquent sites. In the experience reported by Sanai et al., all four patients with permanent postoperative deficits had no positive sites detected prior to their resections.12 Therefore, other authors continue to promote a wider bone flap, in order to obtain a systematic positive mapping before performing the resection.11,12 Moreover, a positive mapping might also allow an optimization of the EOR, since the resection can be pursued until eloquent areas are encountered, that is, with no margin around the functional structures. A recent study demonstrated that in a consecutive and homogeneous series of 115 LGG in the left dominant hemisphere, the rate of permanent deficit remained lower than 2% despite the absence of margin around the language sites.12
IESM allows the mapping of motor function (possibly under general anesthesia, by inducing involuntary motor response, but also in awake patient by eliciting a disturbance of the movement), somatosensory function (by eliciting dysesthesias described by the patient himself intraoperatively), visual function (by eliciting phosphenes and/or visual field deficit described by the patient), auditivo-vestibular function (by inducing vertigo), language (spontaneous speech, counting, object naming, comprehension, writing, reading, bilingualism, switching from one language to another), and also the mapping of higher-order functions such as calculation, memory, spatial cognition, cross-modal judgment or even emotional processing, by generating transient disturbances if the electrical stimulation is applied at the level of a functional “epicenter.”14 It is crucial that a speech therapist/neuropsychologist/neurologist be present in the operative room, in order to interpret accurately the kind of disorders induced by IESM, for instance speech arrest, anarthria, speech apraxia, phonological disturbances, semantic paraphasia, perseveration, anomia, dysculia, and so on. Thus, IESM is able to identify in real-time the cortical sites essential for the function before the beginning of the resection, in order to both select the best surgical approach and to define the cortical limits of the lesion removal.
Another major issue is the use of subcortical mapping throughout the resection, in addition to the cortical mapping before the lesion removal.11,12 Brain lesion studies have taught that damage of the white matter pathways generated more severe deficit than cortical injury. Therefore, the subcortical tracts subserving motor, somatosensory, visual, auditivovestibular, language, and cognitive functions must be detected during the lesion removal, in order to preserve anatomofunctional connectivity while optimizing the EOR, that is, to pursue the resection until eloquent pathways are detected. Interestingly, according to the same principle as that described at the cortical level, IESM can also identify eloquent subcortical structures. It allows the study of anatomofunctional connectivity by directly and regularly stimulating the white matter tracts and deep gray nuclei throughout the resection, and by eliciting functional response when in contact with deep crucial areas (Fig. 6-1). Furthermore, IESM enables a better understanding of the brain connectivity, showing that dynamic cerebral processing is underlain by parallel distributed and interactive networks, the so-called “hodology.”15 This connectionist view also opens the door to the concept of cerebral plasticity, crucial in LGG surgery.
One of the major advantages of IESM for brain mapping in adult patients is that it intrinsically does not cause any false negatives—if the methodology is rigorously applied as detailed previously. Indeed, IESM is highly sensitive for detecting the cortical and axonal eloquent structures, and it also provides a unique opportunity to study brain connectivity, since each area responsive to stimulation is in fact an input gate into a large-scale network, rather than an isolated discrete functional site. IESM, however, also has a limitation, as its specificity is suboptimal. Indeed, IESM may lead to interpretation that a structure is crucial, due to the induction of a transient functional response when stimulated, whereas this effect is caused by backward spreading of the electrostimulation along the network to an essential area, and/or the stimulated region can be functionally compensated thanks to long-term brain plasticity mechanisms. In brief, although IESM is still the gold standard for brain mapping, due to the risk of “false positives,” its combination with new methods such as perioperative FNI and biomathematical modeling is now mandatory, to clearly differentiate those networks that are actually indispensable to function from those that can be compensated.16
IESM: New Insights into Dynamic Functional Organization of the Brain
Anatomofunctional Organization of Supplementary Motor Area
The supplementary motor area (SMA), namely the frontomesial area located in front of the primary motor area of the inferior limb, is involved in the planning of the movement. Its resection induces the classical “SMA syndrome.” This syndrome is characterized by a complete akinesia and even mutism in cases of lesions of the left dominant SMA, which occurs approximately 30 min following the end of the resection, as observed in awake patients. Then, this syndrome suddenly and spontaneously resolves around the 10th day following surgery, even if some rehabilitation is often needed during 1 to 3 months in order to allow a truly complete functional recovery. Using preoperative fMRI, it has been shown that the occurrence of this syndrome was not related to the volume of the frontal resection, but directly to the removal of a specific structure called the “SMA-proper,” detectable on the preoperative FNI. Thus, on the basis of the presurgical fMRI, it is now possible to predict, before surgery, if an SMA syndrome will occur or not postoperatively, and to inform the patient and his family.17 Moreover, by coupling preoperative fMRI, the pattern of clinical deficit after surgery, and the extent of resection on the postoperative MRI, the existence of a somatotopy within the SMA-proper has been demonstrated—namely, from anterior to posterior: the representation of language (at least in the dominant hemisphere), of the face, then the superior limb, and then the inferior limb (immediately in front of the paracentral lobule). As a consequence, it is also possible to predict before SMA resection the severity and the pattern of the postoperative transient deficit (e.g., only mutism, or mutism and akinesia of the superior limb, or akinesia of the entire hemibody). This has an important impact in planning rehabilitation.
Role of Insula in Language and Swallowing
Although the insular lobe is also frequently involved in tumors, particularly LGGs, this structure was poorly studied over a long period of time for technical reasons. In fact, the insula is an anatomical, cytoarchitectonic, and functional interface between the allocortex and neocortex. Recent FNI studies have enhanced understanding of this multimodal lobe in many functions, in particular in language. Indeed, preoperative fMRI has regularly showed an activation of the anterior insular cortex in the dominant hemisphere during language tasks, as reported in healthy volunteers. Moreover, these results were confirmed by IESM, which induced language disorders, and more specifically articulatory disturbances when applied on the insular cortex, supporting the role of this structure in the complex planning of speech, as previously suggested in stroke studies.18 These data have important implications for the neurosurgeon, since in left dominant (frontotemporo) insular lesions, resection carries a high risk of being incomplete. Moreover, following resection of LGG involving the right nondominant insulo-opercular structures, the induction of a transient Foix-Chavany-Marie syndrome can be observed, that is, a bilateral facio-linguo-pharyngo-laryngal palsy, with a reversible inability for the patient to speak and swallow.
Functional Organization of Broca’s Area
Using IESM, it was shown that the classical “Broca’s area” was not basically involved in speech production, but was in fact implied in several language processings: its posterior part (pars opercularis) is more involved in phonological processing, its superior part (pars triangularis) is implied in syntactic processing, and its anterior part (pars orbitaris) is more involved in a large semantic network that overlays the inferior fronto-occipital fasciculus.19 Interestingly, these results provided by IESM are in accordance with those obtained using fMRI, as shown in a recent meta-analysis of the literature.5
Role of Premotor Cortex in Language
Although many studies have allowed a better clarification of the implication of this structure in motor function, its participation in language remains poorly understood. Interestingly, it has demonstrated using IESM that stimulation of the dominant dorsal premotor area (namely the structure lateral to the SMA, in front of the primary motor area of the hand), induced anomia when stimulated. On the other hand, stimulation of the dominant ventral premotor cortex regularly elicited anarthria.12 These results give strong arguments in favor of the involvement of the dorsal premotor cortex in the naming network, in accordance with fMRI studies which have suggested that this region could participate to lexical retrieval and that its engagement might be related to conceptual category; and the involvement of the ventral premotor cortex in the planning of articulation, explaining why lesion studies have reported that damage of the “lower motor cortex” induced speech apraxia (i.e., aphemia).
Functional Organization of Wernicke’s Area
Concerning lesions located within dominant temporal posterior areas, tasks adapted to test comprehension during IESM have been developed. For instance, a triad of pictures is shown, and the patient is asked to pair them by naming two pictures with conceptual links, such as a pyramid and a palm tree. Interestingly, several sites within the posterior part of the superior temporal gyrus specifically elicited an anomia without comprehension disorders when stimulated, although other sites within the same gyrus elicited only comprehension disorders with preservation of the ability to name, and other areas generated only phonological disturbances. These results give some arguments in favor of the complexity of the functional organization of Wernicke’s area (in accordance with fMRI results) with its participation not only in comprehension, but also in naming phonological processing.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree