Chapter 63 Contemporary Dorsal Rhizotomy Surgery for the Treatment of Spasticity in Childhood
Cerebral palsy is a movement disorder resulting from an insult to the immature brain. As a result of the cerebral lesion, there are peripheral motor manifestations such as muscle contractures and joint deformities. Traditionally the contractures and joint abnormalities have been dealt with by physical therapists and orthopedic surgeons. Yet, their success has been limited as they have not addressed the primary pathology. Because the primary pathology is within the nervous system, neurosurgeons have attempted to treat the disorder closer to its source. Although there are many abnormal features associated with cerebral palsy, spasticity has been the only one accessible to neurosurgical techniques. As survival rates have improved among very low birth-weight infants, the incidence of cerebral palsy has increased to 1 in every 500 live births. Currently, cerebral palsy is estimated to affect 500,000 people in the United States alone.1
Spasticity is characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks resulting from hyperexcitability of the stretch reflex.2 It is recognized by its characteristic “clasp-knife” quality during passive limb motion. Although over the past several decades, a variety of treatments have been developed for reduction of spasticity, more recently selective dorsal rhizotomy has been widely and successfully used in treatment of spastic cerebral palsy. Here we describe the operative technique for selective dorsal root rhizotomy.
History of Selective Posterior Rhizotomy
Sherrington in his classic studies on the neurophysiologic basis of muscle tone and spasticity, divided the brain stem from the spinal cord in cats and found that they became spastic. He then opened the spinal canal, cut the posterior rootlets and the spasticity was abolished.3
This work was applied to humans by Foerster in 1905, when he first performed the dorsal (sensory) rhizotomy in four patients. Subsequently, in 1913, he expanded his use of the dorsal rhizotomy to 159 patients.4 Foerster used electrical stimulation during the surgery to identify the level of the nerve roots. He described division of whole posterior nerve roots from L2 to S2, with sparing of L4 and L3 sensory roots to preserve sensation and quadriceps tone for standing. Although he was able to relieve spasticity, the dorsal rhizotomy was not widely used for the next 60 years.
In 1967, Gros5 modified the technique to a partial posterior rhizotomy by saving one or two rootlets in each root to safeguard proprioception. Gros demonstrated that his more conservative procedure resulted in the same beneficial reduction of spasticity. Gros’s “partial cutting” procedure, however, did not adequately predict and target which muscles would be affected by the surgery. To improve the outcomes, Gros’s associates subsequently made adjustments to the rhizotomy procedure.
In 1972, Sindou published anatomic documentation of the location of the 1A sensory fibers of nerve roots (believed to be responsible for spasticity) where they entered the spinal cord.6 In 1975, based on the Sindou thesis, Fraioli and Guidetti further modified the rhizotomy to sever these 1A sensory fibers and preserve other sensory fibers.7 In 1977, Fraioli and Guidetti7 modified the technique to a hemisection of nerve rootlets.
Fasano and colleagues published a method of selective posterior rhizotomy based on electromyographic responses to electrical stimulation.10,11 Working at the level of the conus, they stimulated the dorsal spinal rootlets at increasing frequencies while recording the responses in corresponding anterior roots and muscles. This intervention, the “functional dorsal rhizotomy,” widened understanding of abnormal responses and identified which rootlets should be cut. This system of identification and selective cutting to preserve proprioceptive sensation remains the basis of current surgical rhizotomy procedures.
Operation at the level of the conus medullaris made identification of the lower sacral rootlets involved in bowel and bladder function difficult. In 1981, Peacock modified the procedure by shifting the site of surgery from the conus medullaris region used by Fasano to the cauda equina region.12,13 This shift allowed for easier identification and preservation of both the S3 and S4 nerve roots, as well as separation of individual rootlets of the ventral and dorsal roots. In a consecutive series of 105 children with spastic cerebral palsy who underwent selective dorsal rhizotomy, wound infection, cerebrospinal fluid leak, hemorrhage, and bowel or bladder disturbance were absent.14 In a 20-year follow-up of his original series from Cape Town, 60 patients were shown to have maintained a decrease in muscle tone, increased range of motion, and improved function, including gait.15
Selective dorsal rhizotomy has proven to be a safe and effective procedure for treatment of spasticity, with long lasting results when performed in a methodical and careful fashion.14,15
Patient Selection
Proper patient selection is critical to the desired reduction of spasticity from selective dorsal rhizotomy. First, spasticity must be confirmed and differentiated from other abnormal movement patterns or dystonia.16 Next all of the factors affecting the child’s motor function should be identified and the role of spasticity considered in the overall motor disability. A team approach to evaluation and treatment is used involving a pediatric neurosurgeon, physical therapist, orthopedic surgeon, physiatrist, pediatric neurologist, and developmental pediatrician.
Spasticity can also mask underlying motor weakness or poor control of trunk muscles. The child’s unsupported sitting posture is observed. Inability to sit upright can indicate weakness of the trunk muscles. The child’s reaction to disturbances of equilibrium while sitting are also noted. Only then is antigravity control of the trunk and lower extremities best evaluated. The child’s ability to control body weight while standing upright and with unilateral standing is assessed. Evaluation of flexion and extension ability at the hip, knee, and ankle in unilateral standing is also a useful estimate of motor strength.16
In summary, the children who are appropriate candidates for selective dorsal rhizotomy fall into two groups, severely affected quadriplegic patients with little or no independent function and spastic diplegic children who are walking, with or without support. In the first group of children, the goals of surgery are improved comfort, positioning, and ease of positioning and transport by the caregiver. In spastic diplegics the goal of surgery is improving functional skills including gait and decreasing the risk of developing flexion contractures with the need for orthopedic intervention. Ambulatory patients who have poor trunk control, or poor lower extremity antigravity control, are poor surgical candidates. They are unlikely to have significant benefit from this procedure. The ideal candidates are spastic diplegic children between 4 to 6 years of age who walk independently but with an abnormal gait pattern (due to excessive hip adduction, a flexed hip and knee posture, limited range of hip and knee motion, and an equine posture of the foot and ankle). These children typically have good trunk control and antigravity muscle strength in the hip abductors which are crucial for stability in standing and walking.16
Procedure
The skin is prepped in the usual sterile fashion. Two screens are placed to allow the head and the lower extremities to be visible to the anesthesiologist and the physiologist, respectively, during the procedure. The lower portion of the drapes is placed over a Mayo table so that the physiologist will be able to see and feel for muscle contractions and clonus in the lower extremities during the procedure. Needle electrodes for electromyography are placed in five lower extremity muscle groups bilaterally: adductor, quadriceps, tibialis anterior, hamstrings, and gastrocnemius. Electrodes are also placed in the external anal sphincter so that reflex arcs involved in sphincter control can be detected.
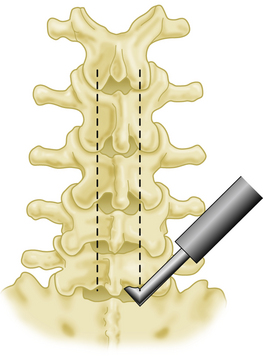
Using a #15 skin knife the ligamentum flavum is opened at the inferior margin of the L5 lamina, and entrance in the epidural space is confirmed by visualization of fat or dura. A Penfield #4 can be used to confirm entry into the epidural space as well, but care must be taken not to cause any bleeding from epidural veins. The footplate of a small craniotome is inserted and brought up against the inferior margin of the lamina of L5, halfway between the spinous process and the facet. Care is taken not to injure the facets. A multilevel laminotomy is performed from L5 up to L2 in one step (Fig. 63-1). The same process is replicated on the opposite side. The supraspinous and interspinous ligaments are divided between L5 and S1 and the inferior portion of the laminar plate is then gently lifted up towards the cephalad extent of the surgical incision (Fig. 63-2). First, drill holes are placed at the base of the spinous process on either side at each level. Then, drill holes are placed in the lateral portions of the lamina at each level and threaded with non-absorbable sutures, each of which is held out laterally with a small hemostat. Then the plate is secured with suture and mosquito hemostats to the drapes superiorly. The lamina of S1 is cut on either side of the spinous process using a thin angled bone rongeur, with the supraspinous ligament left intact (Fig. 63-3). A suture is placed through the spine, which is then reflected caudally to expose the upper portion of the sacral dura. Bone bleeding is controlled with a slurry made from fibrin and Avatin. Bone wax is used to stop any persistent bone bleeding. Use of bone wax is minimized, as it can interfere with bone healing. Bleeding from epidural veins is controlled with bipolar coagulation.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree