Fig. 21.1
(a) Sagittal T1-weighted pre-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A large mass with heterogeneous signal intensity is centered in the sella with a large cystic suprasellar component extending to the foramen of Monro causing hydrocephalus. There are also hypointense structures within the inferior sellar component of the lesion that may represent calcifications. The clivus is eroded
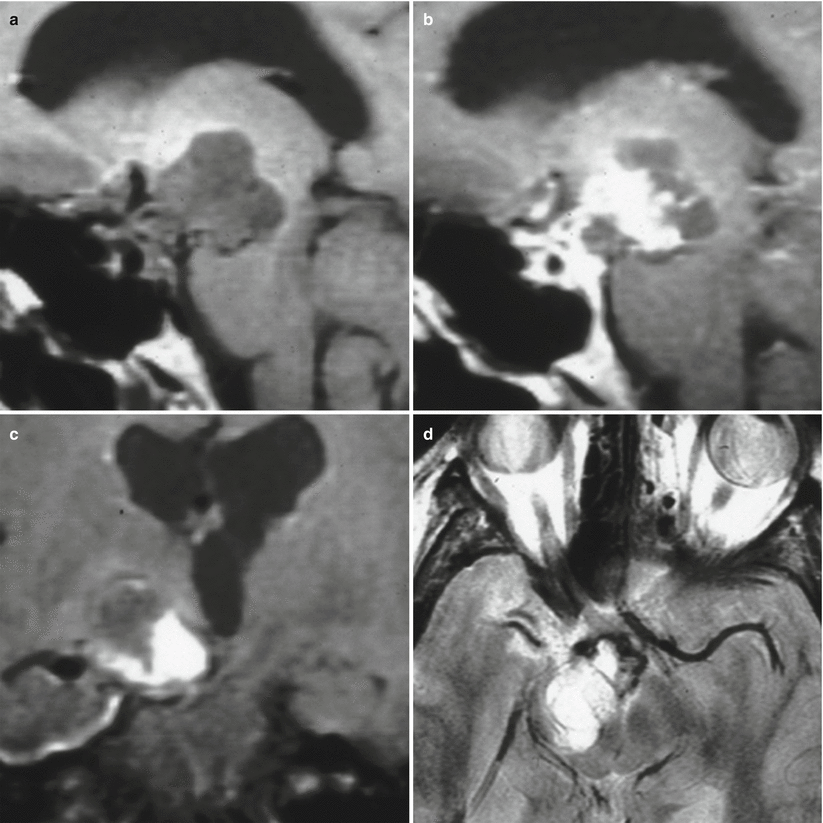
Fig. 21.2
(a) Sagittal T1-weighted pre-gadolinium image. (b) Sagittal T1-weighted post-gadolinium image. (c) Coronal T1-weighted pre-gadolinium image. (d) Axial T2-weighted image. There is a large mixed cystic and solid enhancing mass in the right suprasellar region impressing on the right hypothalamus. Posteriorly, the mass indents on the right midbrain
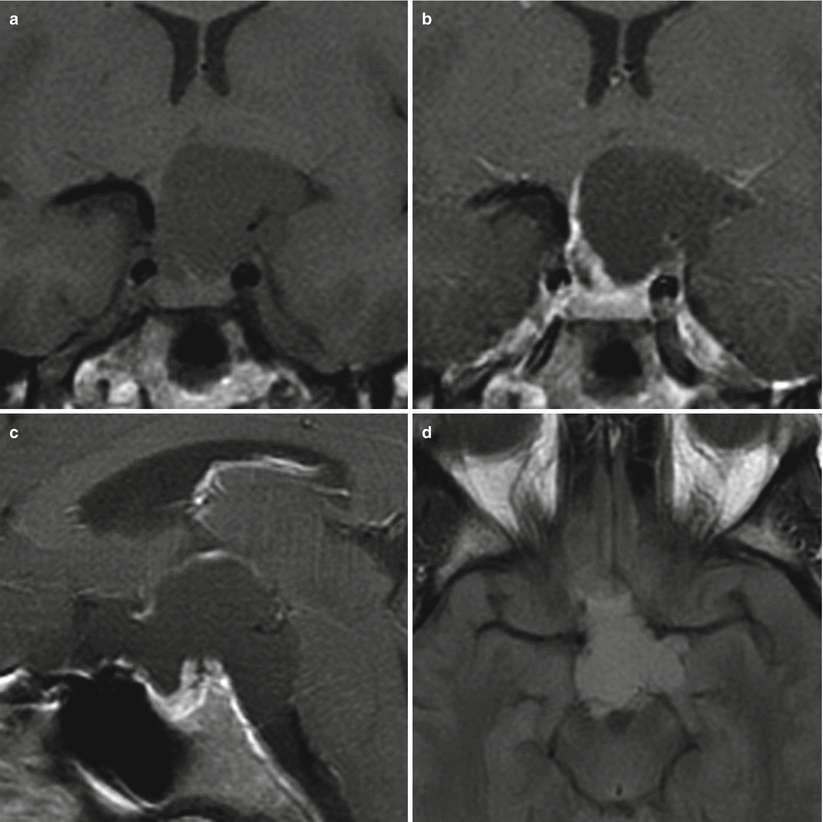
Fig. 21.3
(a) Coronal T1-weighted pre-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. (c) Sagittal T1-weighted post-gadolinium image. (d) Axial fluid-attenuated inversion recovery (FLAIR) image. A large cystic mass centers in the suprasellar region extending to the interpeduncular cistern. The content of the cystic component is slightly hyperintense on both T1-weighted and FLAIR images. The mass indents on the left hypothalamus. The pituitary gland is located in the sella inferior to the mass
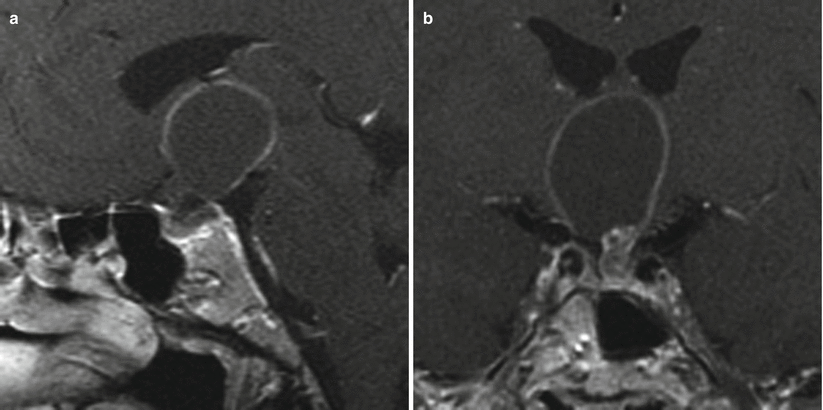
Fig. 21.4
(a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is an enhancing sellar mass with a cystic suprasellar component extending to the root of the third ventricle
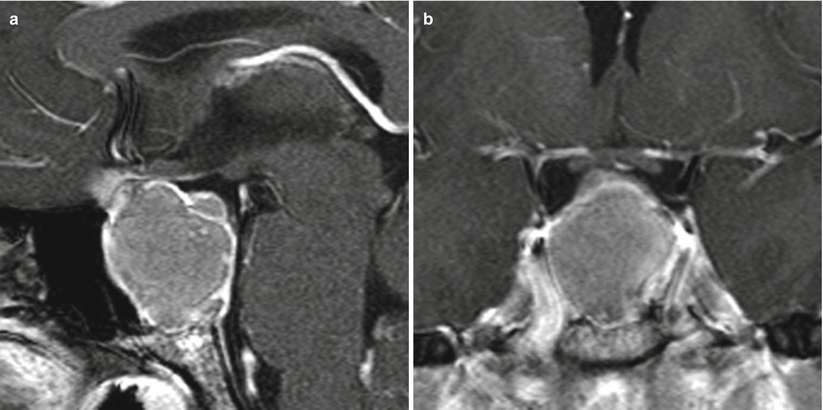
Fig. 21.5
(a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A heterogeneously enhancing sellar/suprasellar mass erodes the sellar floor and contacts the optic chiasm without significant displacement
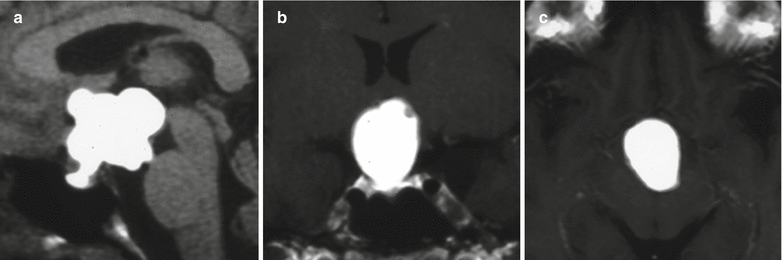
Fig. 21.6
(a) Sagittal T1-weighted pre-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. (c) Axial T1-weighted post-gadolinium image. A lobulated cystic mass centers in the sellar and suprasellar regions. The content of the cystic component is hyperintense on T1-weighted images. The mass extends superiorly to the third ventricle and posteriorly to the interpeduncular cistern
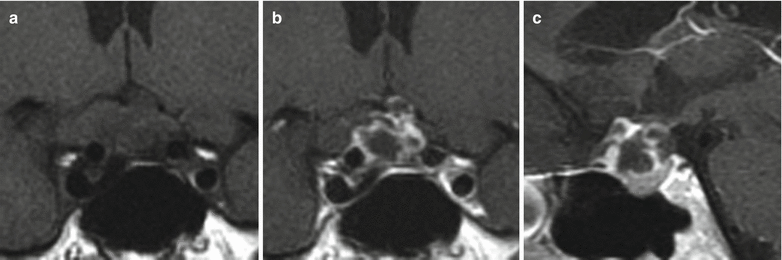
Fig. 21.7
(a) Coronal T1-weighted pre-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. (c) Sagittal T1-weighted post-gadolinium image. There is a mixed cystic and solid enhancing mass in the sellar and suprasellar regions. There is remodeling of the sellar floor. The mass abuts the optic chiasm without significant displacement. There is no encasement of the cavernous internal carotid arteries
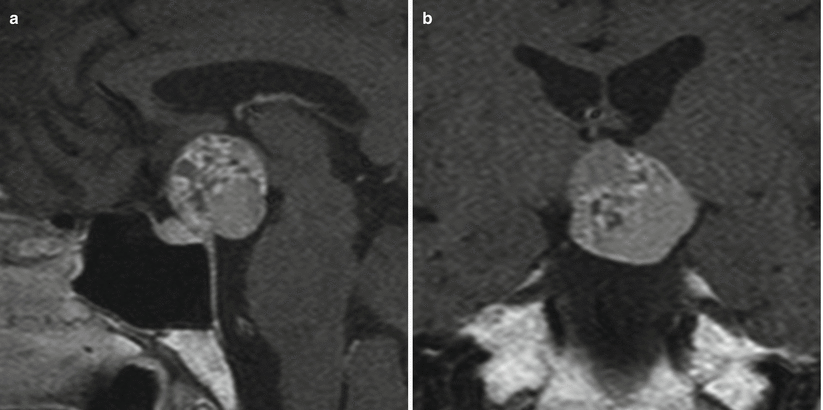
Fig. 21.8
(a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A suprasellar mass with heterogeneous signal intensity extends to the interpeduncular cistern and indents the hypothalami. The pituitary gland is located in the sella inferior to the mass
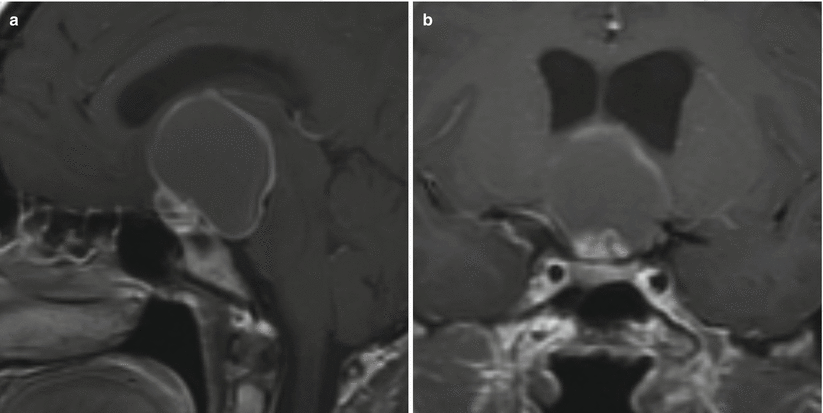
Fig. 21.9
(a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is a suprasellar cystic mass extending to the interpeduncular cistern. The mass indents the hypothalamus. The pituitary gland is located in the sella inferior to the mass
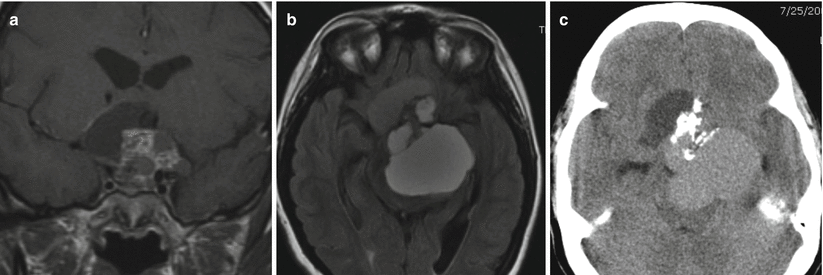
Fig. 21.10
(a) Coronal T1-weighted pre-gadolinium image. (b) Axial fluid-attenuated inversion recovery (FLAIR) image. (c) Axial CT image. A large mixed cystic and solid enhancing sellar and suprasellar mass containing calcification impresses on the hypothalami and brainstem
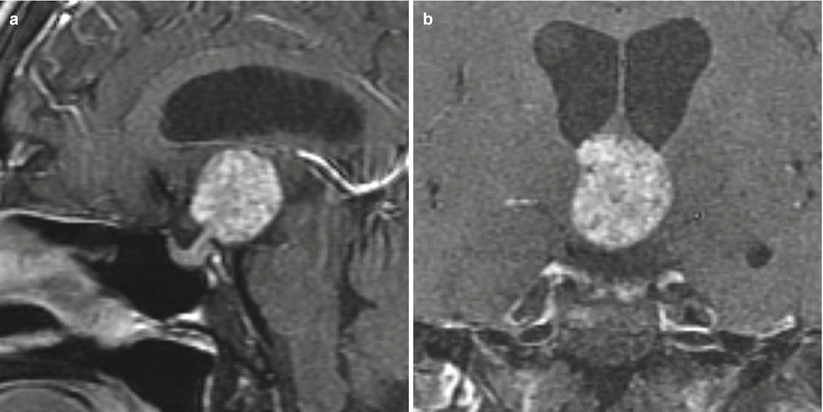
Fig. 21.11
(a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is a mass with heterogeneous signal intensity in the suprasellar region anterior to the third ventricle and abutting the hypothalamus. The pituitary gland and stalk are visualized separately from the mass
21.2.2 Papillary Craniopharyngiomas
The squamous-papillary subtype is found in approximately one third of adult CP cases and rarely shows calcification [4, 18, 20, 23].
The majority of squamous-papillary CPs are predominantly solid or mixed solid-cystic tumors with a spherical shape. They usually exhibit low intensity on T1-weighted images, high intensity on T2-weighted images, and enhancement of the cyst wall after addition of gadolinium [4, 18, 23].
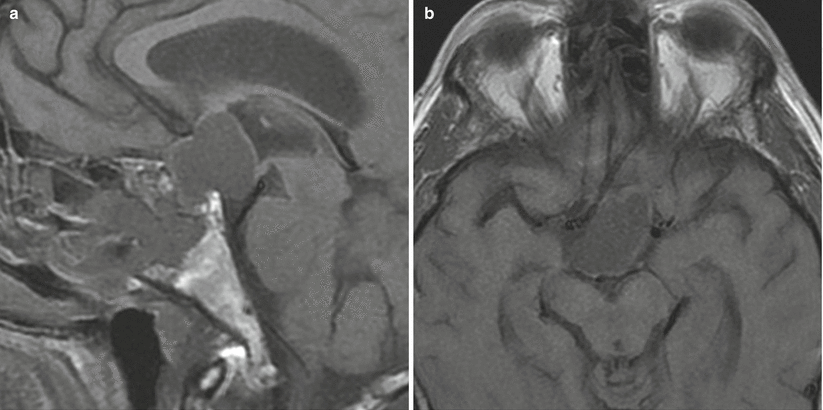
Fig. 21.12
(a) Sagittal T1-weighted pre-gadolinium image. (b) Axial T1-weighted pre-gadolinium image. A mass with heterogeneous signal intensity in the sellar/suprasellar region extends to the interpeduncular cistern. The lamina terminalis is posteriorly displaced. There is erosion of the sella
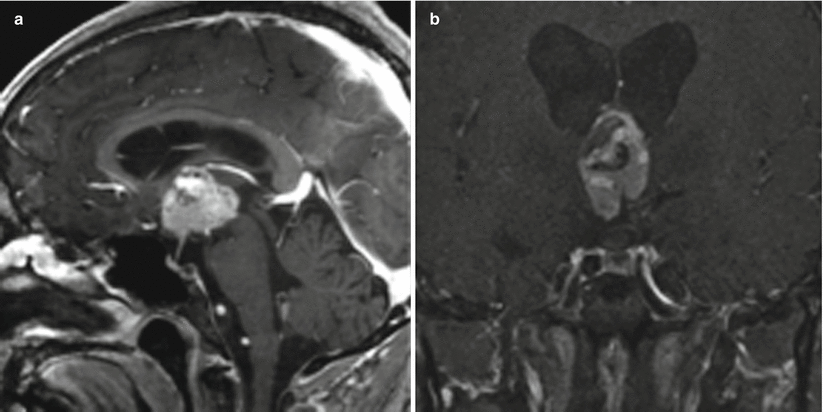
Fig. 21.13
Sagittal (a) and coronal (b) T1-weighted post-gadolinium imaging showing a large, heterogeneously enhancing papillary craniopharyngioma contained entirely within the third ventricle
21.3 Pathology
Many authors have suggested that CPs and Rathke cleft cysts are related lesions representing two ends of a spectrum of similar disease entities originating from ectodermal remnants of Rathke’s pouch [21, 25, 26].
In addition to the two major pathological subtypes (adamantinomatous and papillary), several mixed or transitional forms also have been reported.
Several investigators have argued that adamantinomatous and papillary CPs may represent two distinct entities that are located at opposing ends of a pathological continuum [20, 27].
New molecular genetics data have shown that these tumors may have a different pathway of formation with mutually exclusive gene mutations. Mutations in the CTNNB1 (β-catenin) gene are present in adamantinomatous craniopharyngiomas, whereas recurrent mutations in the BRAF gene are seen in papillary craniopharyngiomas [28].
There have been rare reports of progression to malignancy, mainly in the adamantinomatous variety.
21.3.1 Adamantinomatous Craniopharyngiomas
Adamantinomatous CPs are thought to arise from squamous embryonic rests and bear similarity to adamantinomas or ameloblastomas of the jaw, with the potential for enamel production.
On gross examination, the cystic components are often described as having a characteristic “machine-oil” interior, containing desquamated squamous epithelium and comprising mainly keratin and cholesterol.
The solid component has a rubbery texture and may contain foci of calcifications or even bone.
Histologically, the tumors are characterized by cystic formations of stratified epithelium with a palisading arrangement of basal cells, intermixed with nodular strands of solid and cystic epithelium, which in turn undergo transition to loose tissue termed stellate reticulum and “wet keratin” (Fig. 21.14).
The nodular masses of “wet keratin” are distinctive and diagnostic of the adamantinomatous variant.
Extensive fibrosis and xanthogranulomatous reaction with chronic inflammation and cholesterol clefts with a giant cell reaction may be prominent.
CPs may compress or invade the brain parenchyma, producing variable gliosis and occasional Rosenthal fiber formation that may mimic pilocytic astrocytoma.
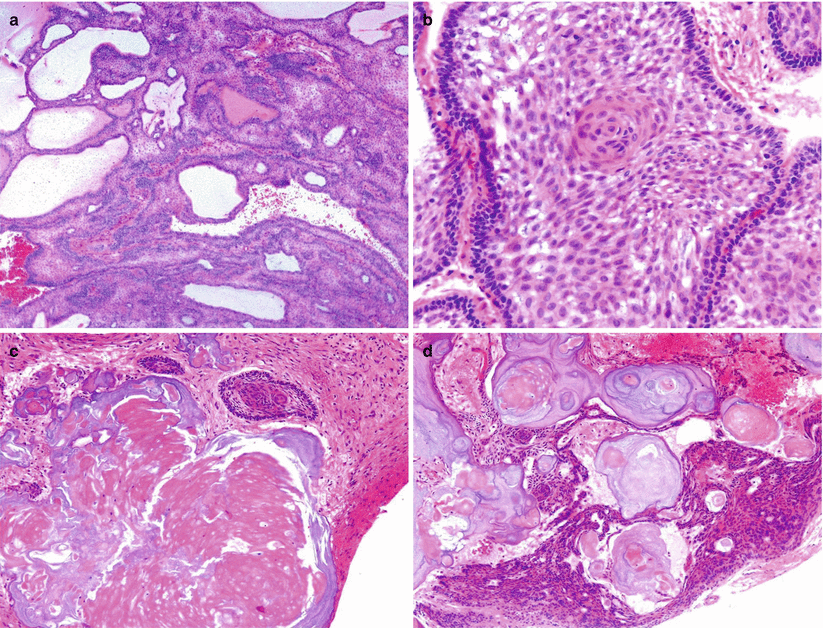
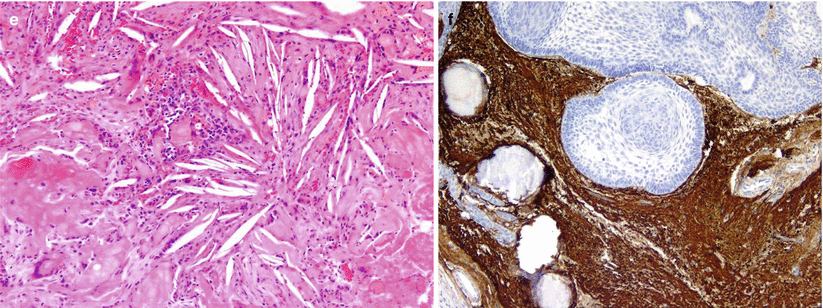
Fig. 21.14
Adamantinomatous craniopharyngioma (CP). Adamantinomatous CP typically shows a stratified epithelium with a palisading arrangement of the basal cells that mature into large, flat cells within a loose stroma (hematoxylin and eosin [H&E] stain) (a, b). One of the most characteristic features of adamantinomatous CP is the formation of “wet keratin” consisting of ghost keratinocytes with no nuclei (c). Adamantinomatous CP exhibits cystic formation (a) and calcifications (d). Reactive inflammatory reaction is commonly seen, with xanthomatous changes including the presence of large macrophages and cholesterol cleft accumulation (e). Intense gliosis in the adjacent brain parenchyma can be mistaken for low-grade glioma and highlighted by GFAP immunostain (f)
21.3.2 Papillary Craniopharyngiomas
Papillary CPs are more common in adults (14–50 % of CPs in adults, compared with 2 % in children) [4].
Papillary CPs tend to be more solid circumscribed masses that lack the typical calcification and “machinery oil” cyst content seen in adamantinomatous tumors.
The tumors often involve or sit within the third ventricle.
Histologically, the tumor consists of well-differentiated, squamous-lined papillae surrounding cores of fibrovascular stroma (Fig. 21.15).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








