Study
Type
No. of patients
Seizure type
Follow up
Stimulation parameters
Resultsa
SANTE
Multi-center, double-blind, randomized controlled trial
108
Partial onset
3 months (blinded)
145 Hz, 1 min on, 5 min off (blinded)
29 % increased reduction in active vs. control groups (blinded)
Fisher et al. (2010)
25 months (unblinded)
variable (unblinded)
56 % reduction (unblinded)
Osorio et al. (2007)
Case series
4
Mesiotemporal
36 months
157 Hz (mean), 1 min on, 5 min off
75 % reduction
Lim et al. (2007)
Case series
4
Heterogeneous
33–48 months
90–110 Hz, continuous and cycling
51 % reduction
Hodaie et al. (2002)
Case series
6
Heterogeneous
11–21 months (short-term)
100 Hz, 1 min on, 5 min off
53.8 % reduction (short-term)
Andrade et al. (2006)
2–7 years (long-term)
No change long-term
Lee et al. (2006)
Case series
3
Heterogeneous
2–10 months
130 Hz, 1 min on, 5 min off
75.4 % reduction
Kerrigan et al. (2004)
Case series
5
Partial onset
12 months
100 Hz, 1 min on, 5 min off
Statistically significant reduction in 1/5 patients
Cooper et al. (1984)
Case series
6
Partial onset
3 years
60–70 Hz, continuous
Clinically significant improvement in 4/6 patients
Although the ANT is a relatively large structure, one study suggests that the internal complexity of the thalamus may make effective targeting difficult (Osorio et al. 2007). They recorded in bilateral hippocampi during bilateral ANT stimulation in four patients, with highly variable responses across patients. They also confirmed placement with post-operative MRI and found that only three of the 16 stimulating contacts (two per nucleus in four patients) were located in the ANT proper. Curiously, the patient with contacts in the ANT had the most modest reduction in seizures with stimulation.
Since ANT lesions have themselves been shown to reduce seizures (Mullan et al. 1967), the question of a “lesion effect” from stimulator placement is also important. Hodaie et al. (2002) reported an immediate mean reduction in seizure frequency of 56.8 % after electrode implantation compared to baseline. They found no significant change in seizure reduction out to an average of 15 months when the stimulators were turned on, nor when they were turned off for a period of 2 months in a blinded fashion. These patients were subsequently followed for 2–7 years, during which time seizure frequency reductions over baseline were maintained, but did not improve (Andrade et al. 2006). In another case series, however, some individual patients were noted to suffer an increase in seizure frequency when the stimulators were unintentionally turned off (without awareness of the patient), with return of seizure improvement with resumption of stimulation (Kerrigan et al. 2004). These results only emphasized that a randomized, controlled, blinded study was needed.
In 2010 the anticipated results of the multi-center, double-blind SANTE trial (Fisher et al. 2010) were published. To be included in this trial patients had to have partial seizures, with or without secondary generalization, in line with the logic that ANT stimulation disrupts subcortical–cortical propagation of seizures along a distinct limbic pathway. Patients had failed at least three antiepileptic drugs and had no evidence of a structural brain lesion. Patients were implanted with bilateral ANT stimulating electrodes after completing a 3-month baseline period during which their antiepileptic drug regimen remained stable. If an electrode was not within the ANT on post-operative imaging, it was replaced. Patients were randomized to stimulation on or stimulation off groups 4 weeks after implantation. The blinded phase lasted 3 months and included 108 patients. Using a model that adjusted for repeated measurements, the authors reported a 29 % greater seizure frequency reduction in the stimulation group compared to the control group during month 3 of the blinded phase. It is instructive to look at the (unadjusted) median seizure frequency improvement in the blinded phase over time (Fig. 14.1). Both the stimulation and the control groups saw a comparable reduction in seizures in the month after implantation, before stimulation, which may capture a conjectured “lesional effect.” In the ensuing blinded months, however, the two groups progressively separate, the difference becoming significant in the third month. After the 3-month blinded phase, all patients entered an open trial of stimulation with restrictions on stimulation parameters. After month 13 stimulation parameters were allowed to vary freely. Median seizure frequency reduction at 13 months was 41 %, and increased to 56 % at 25 months. These results demonstrate a benefit of high-frequency bilateral ANT stimulation for refractory epilepsy. Further, more than having failed antiepileptic medication, 44.5 % of patients had failed vagal nerve stimulation, and 24.5 % had achieved no benefit from surgical resection, demonstrating that ANT DBS may be the therapy of choice in certain patients with partial epilepsy. Further trials need to be done in patients with primary generalized seizures, such as those with epilepsy syndromes (e.g. West syndrome, Lennox-Gastaut syndrome), who are often among the most difficult patients to treat (Sander 2003).
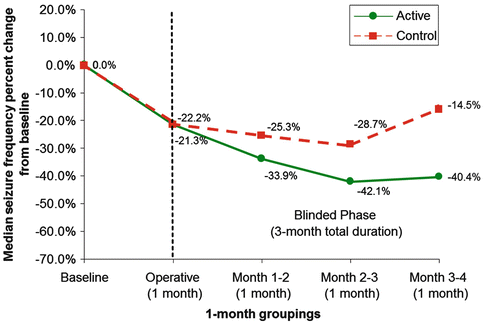

Fig. 14.1
SANTE trial: electrical stimulation of the anterior nucleus of thalamus for the treatment of refractory epilepsy. The graph shows unadjusted median total seizure frequency percent change from baseline by 1‐month groupings and treatment group during the blinded phase. Patients (n = 108) included in this graph were those with at least 70 diary days in the blinded phase (including the outlier). The operative data point contains cumulative data from hospital discharge to 1 month post-implantation but prior to randomization (no active stimulation). Month 1–2 contains cumulative data from month 1 visit to month 2 visit. Month 2–3 contains cumulative data from month 2 visit to month 3 visit. Month 3–4 contains cumulative data from month 3 visit to month 4 visit (From Fisher et al. (2010), with permission)
14.4 Target: Hippocampus
Whereas the idea behind ANT stimulation is to disrupt seizure propagation through a limbic network, hippocampal stimulation is aimed at disrupting the epileptogenic focus itself. Mesial temporal lobe epilepsy is the most common of the medically refractory chronic epilepsies. When there is a single, identifiable electrographic focus, these patients do well with removal of that focus (i.e. temporal lobectomy), 66 % of whom achieve long-term seizure freedom (Tellez-Zenteno et al. 2007). However, many of these patients have bilateral seizure foci, or develop contralateral foci after resection and thereby remain refractory. Furthermore, memory decline after resection can be substantial, particularly in those who undergo dominant temporal lobe resection, or who continue seizing postoperatively (Helmstaedter et al. 2003). Thus, there is a subpopulation of patients with refractory mesial temporal epilepsy for whom resective surgery is not an option, who may respond to epileptogenic disruption via hippocampal DBS.
Velasco et al. (2000) did much of the original groundwork by taking advantage of ten patients undergoing hippocampal depth electrode placement for temporal lobectomy candidacy evaluation. After identifying the area of ictal onset within the hippocampus, recording electrodes were removed and chronic stimulating electrodes implanted along the hippocampal axis. They stimulated over 2–3 weeks and demonstrated a reduction in clinical seizures, reduction in interictal spiking, and temporal lobe metabolic normalization. They then examined the resected tissue after temporal lobectomy to confirm that there was no observable tissue damage from stimulation. This study was important because it demonstrated the feasibility of hippocampal stimulation for temporal epilepsy and confirmed that the ability to reduce interictal spiking on EEG correlated with seizure reduction. Indeed, another group of investigators has used the reduction of interictal spiking by at least 50 % as a necessary condition for chronic stimulation with good results (Vonck et al. 2002; Boon et al. 2007). Table 14.2 provides a summary of clinical studies of hippocampal DBS for epilepsy.
Table 14.2
Clinical trial for hippocampus stimulation to treat epilepsy
Study | Type | No. of patients | Seizure type | Follow up | Stimulation parameters | Resultsa |
|---|---|---|---|---|---|---|
McLachlan et al. (2010) | Blinded, cross-over design | 2 | Bilateral temporal onset | 3 months each: stim, washout (no stim), no stim | Bilateral, 185 Hz, 90 μs, variable intensityb, continuous | 33 % reduction (stim vs. no stim) |
Velasco et al. (2007) | Case series with initial blinded control period | 9 | Unilateral or bilateral temporal onset | 1 month (stim vs. no stim), 18 months unblinded | Uni-, or bilateral, 130 Hz, 450 μs, 300 μA, 1 min on, 4 min off | All patients with significant reduction |
Tellez-Zenteno et al. (2006) | Blinded, cross-over design | 4 | Unilateral or bilateral temporal onset | 3 months stim, 3 months no stim | Unilateral, 190 Hz, 90 μs, variable intensityb, continuous | 15 % reduction (stim vs. no stim) |
Boon et al. (2007) Vonck et al. (2002) | Case series | 12 | Unilateralc temporal onset | 5.5–21 months | Unilateralc, 130 Hzd, 450 μs, variable intensityb, continuous | 30–100 % reduction in 11/12 patients |
Velasco et al. (2001) | Case series | 10 | Unilateral or bilateral temporal onset | 16 days | Unilateral, 130 Hz, 450 μs, variable intensityb, continuous | Seizure free in all patients by day 7 |
Velasco et al. (2007) again published a series of nine patients, all with significant seizure reduction after 18 months to 7 years of follow-up. Two further interesting results came of this. First, in a 1-month blinded period after implantation during which some patients remained with their stimulators off, no beneficial “lesion effect” was seen, a result confirmed by other blinded studies (Tellez-Zenteno et al. 2006; McLachlan et al. 2010) and in contrast to ANT DBS studies. This probably reflects the size and complexity of the hippocampus and suggests that in lieu of complete amygdalohippocampectomy, active disruption of local, epileptogenic circuitry may be important in reducing some temporal-origin seizures. Second, Velasco et al. (2007) found that patients with structural mesial temporal sclerosis on MRI had delayed and more modest seizure control when compared to those with a normal MRI. This is the inverse of resective surgery patients, who achieve better seizure control if they have an abnormal MRI (Tellez-Zenteno et al. 2010). Indeed, Vonck et al. (2002) and Boon et al. (2007) in their series required normal MRIs to be a candidate for chronic hippocampal DBS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







