Fig. 9.1
Microelectrode recording of Vim and electromyogram of tremor in 60-year-old male with tremor-dominant Parkinson’s disease. (a) Schematic presentation of trajectory to Vim in Schaltenbrand–Wahren atlas (1977) at 12 mm lateral to midline. Circle on the AC–PC line indicates tentative target at 25 % distance anterior to PC. The trajectory makes an approximately 60° angle from the AC–PC line, passing through the interface of Vop/Vim and the most ventral part of Vim. (b) Microelectrode recording on the trajectory to Vim. The grouping discharges, which were mildly to strongly related to the tremor activity, were observed at various depths to the tentative target. (c) Tremor activity (5 Hz) on the surface electromyogram on the right biceps muscle
9.7 Mechanism of Therapeutic Effect and Microlesion Effect (Microthalamotomy Effect)
The mechanism underlying Vim-DBS is unknown. Since Vim-DBS acts as a reversible thalamotomy, it is postulated that neuronal blocking and jamming are possibilities (Benabid et al. 1996), but the activation of inhibitory mechanisms should also be considered (Strafella et al. 1997). Many tremors tend to be transiently alleviated soon after microelectrode recording or insertion of DBS leads even without lesioning or electrical stimulation, which is called the microlesion effect (MLE) or the microthalamotomy effect (Benabid et al. 1996). The detailed mechanism of MLE is not clear; however, perifocal edema or gliosis around the electrode track are postulated because most of MLE disappear within a few weeks postoperatively (Benabid et al. 1996; Tasker 1998). A few studies showed a relatively long MLE lasting up to 6 months (Sitburana et al. 2010) or more than 1 year (Kondziolka and Lee 2004; Blond et al. 1992). The duration of MLE may depend on the extent of gliosis or tissue reaction (Blond et al. 1992) or the focal metabolism in the cerebello-thalamo-cortical network (Mure et al. 2011). The existence of MLE did not predict the tremor outcome but the stimulation parameters remained lower at 6 months in the marked MLE group (Sitburana et al. 2010).
9.8 Stimulation Parameters
Detailed settings for Vim-DBS can be usually optimized after the MLE disappears. The contact at the ventral Vim is initially selected as a negative polarity (cathode) of unipolar configuration. Typically, a pulse width of 60 μsec and a frequency of 130 Hz are selected. The pulse width required for tremor inhibition was as short as 50 μs (Strafella et al. 1997). The therapeutic effect of Vim-DBS can be obtained at the frequency of 100–1,000 Hz, and the frequency of 130 Hz is generally used for stable tremor relief (Benabid et al. 1996; Limousin-Dowsey 2002). Then the voltage is progressively increased to find the threshold for tremor cessation of the target limb (Limousin-Dowsey 2002). In PD patients, some tolerance and rebound effect can occur. Possible stimulation tolerance during stimulation and tremor rebound at terminating stimulation should be made clear to the candidates for Vim-DBS. Blond et al. (1992) reported the reappearance of tremor in three out of ten PD patients during a mean follow-up period of 19.4 months. There was a significant increase in voltage at 3 months compared to 1 week after surgery and at 6 months compared to 3 months Hariz et al. 1999). Rebound tremor was observed by several studies (Blond et al. 1992; Benabid et al. 1996; Albanese et al. 1999; Kumar et al. 1999). In order to avoid tolerance development, we instruct patients to turn on Vim-DBS when getting up in the morning and terminate it before sleep. If the rebound tremor returns as soon as the DBS is turned off, producing a tremor-induced insomnia, a family member is advised to turn off DBS after the patient falls asleep. On the other hand, a patient with STN-DBS is told to keep DBS on all day long even during sleep, because nocturnal awakening is a frequent problem when DBS is turned off during sleep. Vim-DBS, unlike thalamotomy, selectively reduces tremors without altering sleep or sleep spindles (Arnulf et al. 2000a). STN-DBS showed both subjective and polysomnographic evidence of improvement of sleep (Arnulf et al. 2000b; Hjort et al. 2004). In an empirical observation, the recurrence of tremor in both upper and lower extremities after Vim-DBS can be alleviated by STN-DBS (Fraix et al. 2005), while no patient with STN-DBS has needed to be secondarily implanted in the Vim because of lack or loss of effect, so far.
9.9 Complications
Complications are usually related to incorrectly placed lesions or unnecessarily large lesions (Fox et al. 1991; Jankovic et al. 1995), Vim borders the posterior limb of the internal capsule laterally and the Vc posteriorly. Even within Vim, too laterally placed lesions lead to contralateral hemiparesis and too posteriorly placed lesions lead to paresthesia or numbness of the contralateral fingers and lip corner. Also lateropulsion is frequently but transiently seen in the immediate postoperative period, which may be related to the interruption of cerebellar afferents. Similarly, the lateral current spread from Vim-DBS may result in contralateral muscle rigidity and the posterior current spread may result in dysesthesia. Benabid et al. (1991) recorded paresthesia (9.4 %), dystonia (6.3 %), gait disorders (12.5 %), and dysarthria (21.9 %) in 32 Vim-DBS cases. The side effects related to Vim-DBS were generally mild and well tolerated and acceptable if the DBS significantly alleviated the tremor (Hariz et al. 1999). Because bilateral thalamotomy inevitably results in persistent dysarthria as a complication (Matsumoto et al. 1984), irrespective of staged or contemporaneous surgery, a bilateral Vim-DBS or a combination of unilateral Vim-DBS and contralateral thalamotomy should be recommended for tremor cases of bilateral limbs, head or jaw (Koller et al. 1997; Schuurman et al. 2000). In 44 % of the patients who had a previous thalamotomy, DBS on the contralateral Vim provoked some dysarthria when the stimulation was activated (Hariz et al. 1999). The DBS effect itself is reversible in nature, and the stimulation intensities or parameters should be explored within the extent that does not produce adverse effects. With the recent advancement of a neurostimulator (Activa SC, Medtronic), patients can adjust the stimulation intensity of one or both sides by themselves to find an optimal intensity.
9.10 Vim-DBS Versus Thalamotomy; The Surgical Results in Kaizuka Hospital
In a comparison study by Schuurman et al. (2000, 2008) between the efficacy of unilateral thalamotomy and that of unilateral or bilateral Vim-DBS for a drug-resistant tremor in 45 parkinsonian patients, 93 % of thalamotomy and 88 % of Vim-DBS patients still experienced satisfactory tremor suppression at 5 years. Adverse effects, such as cognitive decline, dysarthria, and gait and balance disturbance were more often seen after thalamotomy (Schuurman et al. 2000), while some DBS-treated patients had hardware-related complications requiring additional surgery (Pollak et al. 2002). Vim-DBS is safer than thalamotomy and can be carried out bilaterally in either one or two stages (Benabid et al. 1991). In Kaizuka Hospital, 35 thalamotomies and 54 Vim-DBS procedures were performed in tremor-dominant Parkinson’s disease cases between April 1993 and February 2004. In this period, thalamotomy was gradually replaced by Vim-DBS because of the nature of safety, reversibility and adjustability in Vim-DBS although the targeting method of Vim-DBS was the same as the method used in thalamotomy. The tentative target coordinates for Vim was determined by an indirect method based on the AC–PC line on MRI findings: 5.5 mm (or 25 % of AC–PC distance) anterior to the PC (Y), 11 mm lateral to the lateral wall of the third ventricle (X) and 0 mm above AC–PC line (Z). The target determination was supplemented by the guidance of microelectrode recording and electromyogram (Fig. 9.1); the tremor neurons were identified by discharges synchronized with tremor activity on electromyogram. The scores of rest tremor in the medical records were available for 22 thalamotomies and 51 Vim-DBS procedures, and the longest follow-up visits were 36 and 48 months, respectively (Fig. 9.2). At the first month postoperatively, excellent results with complete tremor disappearance or decreased tremor score over two points were observed in 15 out of 22 thalamotomies (68.2 %) and 36 out of 51 Vim-DBS (70.6 %). The tremor evaluation after each surgery was terminated when additional surgery was needed due to tremor recurrence or other parkinsonian symptoms. Thalamotomy brought an immediate tremor cessation, but one-third of patients presented the tremor recurrence, most of which were apparent within 1 year. Vim-DBS also produced an immediate and powerful therapeutic effect, and most tremor recurrence in the acute phase was seen within 1 month postoperatively as well (Fig. 9.2), suggesting the resolution of the microlesion (microthalamotomy) effect; however, all of the recurred tremors were attenuated to a level lower than their preoperative tremor score by adjustment of stimulation parameters. In Fig. 9.3, the postoperative courses of tremor severity for 2 years were compared between 14 thalamotomies and 16 Vim-DBS. The therapeutic effect of Vim-DBS was stable for 2 years, while that of thalamotomy were stabilized after 1 year. When tremor recurrence was defined as the deterioration over one point of score from the peak benefit after surgery, the cumulative recurrences of thalamotomy were 18 % at 3 months and 19 % at 1 year postoperatively, while those of Vim-DBS were 6 % at 3 months and 18 % at 1 year (Fig. 9.3c).
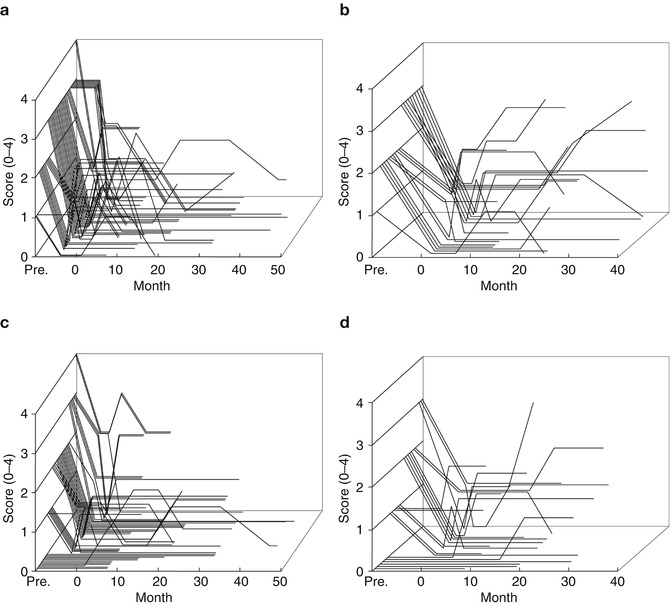
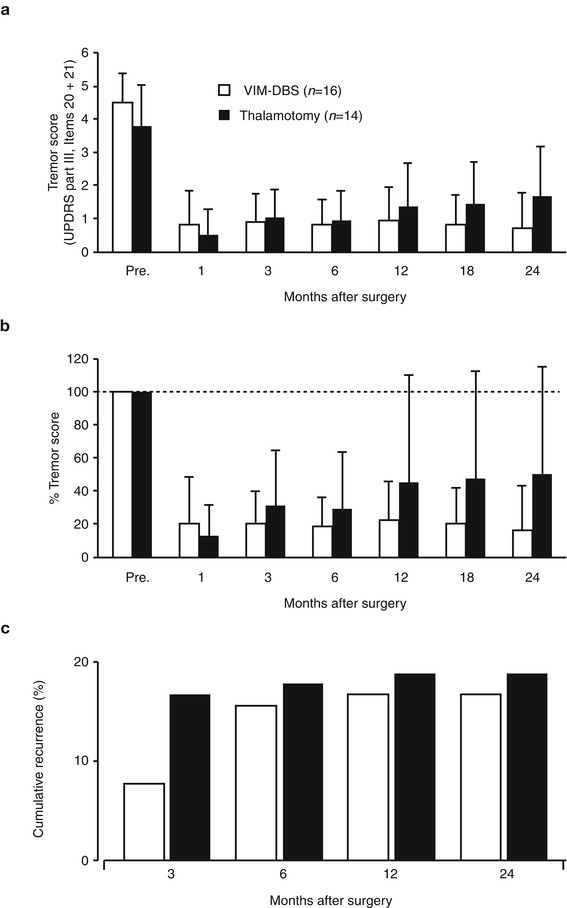

Fig. 9.2
Postoperative change in individual tremor severity in parkinsonian patients. (a, b) Rest tremor, item 20 of UPDRS part 2, (c, d) action or postural tremor, item 21 of UPDRS part 2. (a, c) Vim-DBS (n = 51), (b, d) thalamotomy (n = 22)

Fig. 9.3
Summary of surgical results of Vim-DBS (open column, n = 16) and thalamotomy (closed column, n = 14). Bars indicate standard deviation. (a) Postoperative changes in tremor score (items 20 and 21 of UPDRS part 2). (b) Percent tremor score show the relative changes when the preoperative value was assumed to be 100 %. (c) Cumulative recurrence rate of tremor
9.11 Stimulation Parameters and Stimulation Site of Vim-DBS
Most of the adjustment was the increase in voltage, pulse width, and frequency. Some of the adjustment included the change in polarity (bipolar to unipolar configuration) and multiple cathode contacts. There was a significant increase in voltage at 3 months compared to 1 week after surgery and at 6 months compared to 3 months as also observed by another study (Hariz et al. 1999). The surgical procedure for targeting Vim is the same for thalamotomy and Vim-DBS. Contrary to the immediate surgical results in thalamotomy, in Vim-DBS, the stimulation parameters are inevitably increased from the initial setting in order to attenuate any possible tremor recurrence because the actual intensities required for tremor relief are usually masked by the microlesion effect immediately after the implantation. The spatial relationship between the effective sites of thalamotomy lesion and effective active contact for Vim-DBS is unclear. We have investigated this problem by focusing on the positive results of 6 thalamotomy and 22 Vim-DBS. To determine the Y– and Z-coordinates, the center of the thalamotomy lesion and the active contact for the effective cathode were localized using postoperative MR imaging (Miyagi et al. 2007; Yoshida et al. 2008). As shown in Fig. 9.4, the sagittal sections parallel to the mid-plane, including the AC–PC line and DBS contacts and thalamotomy lesion, were overlaid. The X-coordinates were measured from the lateral wall of the third ventricle on the horizontal section. Both the centers of lesion and effective cathode were located around 12 mm to the lateral wall of the third ventricle, corresponding to the external portion of Vim (Fig. 9.4c) which is adjacent to the pyramidal tract in the posterior limb of the internal capsule. Although the lateral distribution showed no significant difference between lesion center and effective cathode, the centers of effective cathode distributed more posteriorly than the centers of effective lesion. The adjusted energy use (AEU) of the implantable pulse generator at the 2-year follow up showed two peaks in the histogram (Fig. 9.5a): under 20 (low AEU group) and over 20 (high AEU group). The low AEU group was distributed more posteriorly than the centers of lesion (p = 0.003, Fig. 9.5b), while the high AEU group showed the same distribution as effective lesion (Fig. 9.5c). The battery longevities estimated for high and low AEU groups were 5 and 10 years, respectively (Table 9.1). Therefore, when the lower intensity with minimal active contact is assumed to indicate the more favorable results, the effective cathode must locate as posteriorly as adjacent to Vc and as ventrally as close to AC–PC line. This observation is similar to another study of thalamotomy (Atkinson et al. 2002), which found that the lesion including the interface of the Vim with the anterior Vc is necessary for inclusion of proprioceptive thalamus to get an excellent outcome. However, the posteriorly located the thalamotomy lesion, the higher risk for paresthesia is anticipated. On the other hand, the active contacts used for chronic Vim-DBS were significantly anterior and dorsal to the ideal thalamotomy target (Kiss et al. 2003), which may reflect the advantage of adjustability in Vim-DBS. In contrast to thalamotomy lesions, electrodes for Vim-DBS can be arranged in such a way that wide areas can be stimulated (Katayama et al. 2005).
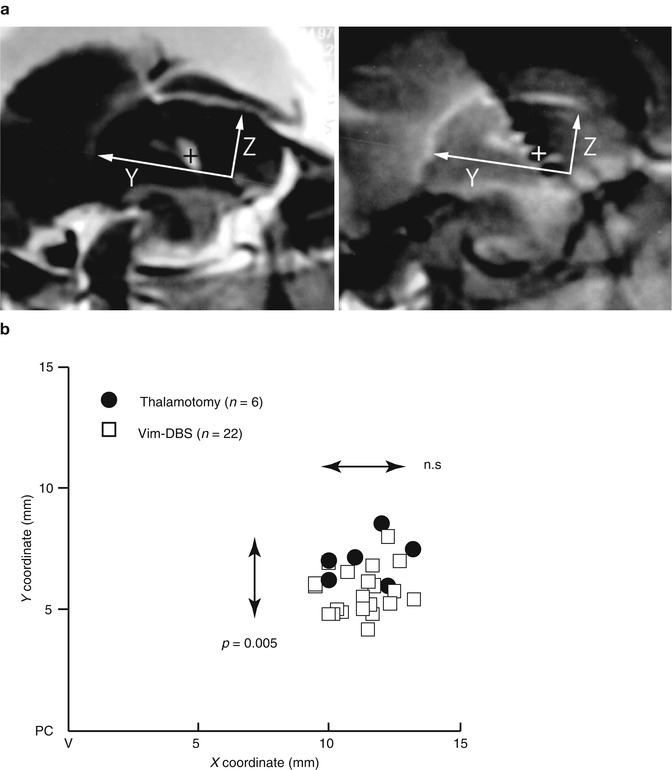
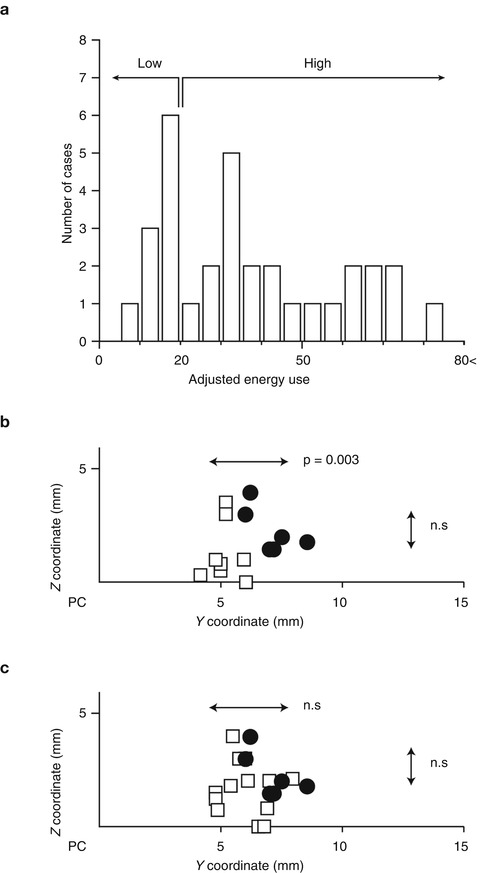

Fig. 9.4
Distribution of centers of effective lesion and effective cathode. (a) Method of measurement of lesion center (left) and DBS cathode (right) by magnetic resonance imaging. The sagittal sections parallel to the mid-plane, including AC–PC line and DBS contacts and thalamotomy lesion, were overlaid (Miyagi et al. 2007; Yoshida et al. 2008). The X-coordinates were measured from the lateral wall of the third ventricle on the horizontal section. The center was measured assuming AC–PC line as Y axis and anterior edge of PC as the origin of a three-dimensional axis. (b) X–Y distribution of centers of effective lesion and effective cathode of the cases showing excellent tremor control. X coordinate was the distance from the lateral wall of the third ventricle (V). Closed circle thalamotomy, open square Vim-DBS. Mann–Whitney U-test detected the significantly posterior distribution of effective cathodes of Vim-DBS (n = 0.005)

Fig. 9.5
Y–Z distribution of centers of effective DBS cathodes and the relationship with adjusted energy use. (a) Histogram of adjusted energy use of Vim-DBS with excellent outcomes. (b, c) X–Y distribution of centers of effective lesion (n = 6) and effective cathode of the cases showing excellent tremor control with low AEU (b, n = 8) and high AEU (c, n = 22). Closed circle thalamotomy, open square Vim-DBS. Mann–Whitney U-test detected the significantly posterior distribution of effective cathodes of Vim-DBS of low AEU group (n = 0.003) but not high AEU group
Table 9.1
Summary of DBS parameters
Adjusted energy use | High, ≥20 (41.4 ± 14.1) | Low, <20 (15.3 ± 3.3) | |
Case number | 14 | 8 | |
Follow up (month) | 40.5 ± 17.8 | 40.8 ± 22.9 | |
Parameter | |||
Pulse width (μsec) | 146.3 ± 63.0 | 78.8 ± 22.3 | |
Frequency (Hz) | 149.4 ± 24.4 | 151.9 ± 18.3 | |
Amplitude (volt) | 2.4 ± 0.5 | 1.7 ± 0.5 | |
Impedance (ohm) | 1218.8 ± 339.7 | 1679.8 ± 301.8 | |
Current (mA) | 2.1 ± 0.3 | 1.1 ± 0.4 | |
Polarity configuration | |||
Bipolar | 1 cathode | 6 (42.9 %) | 7 (87.5 %) |
2 cathodes | 4 (28.6 %) | 0 (0 %) | |
Unipolar | 1 cathode | 3 (21.4 %) | 1 (12.5 %) |
2 cathodes | 1 (7.1 %) | 0 (0 %) | |
Estimated IPG longevity (years) | 5.1 ± 1.1 | 10.0 ± 1.3 | |
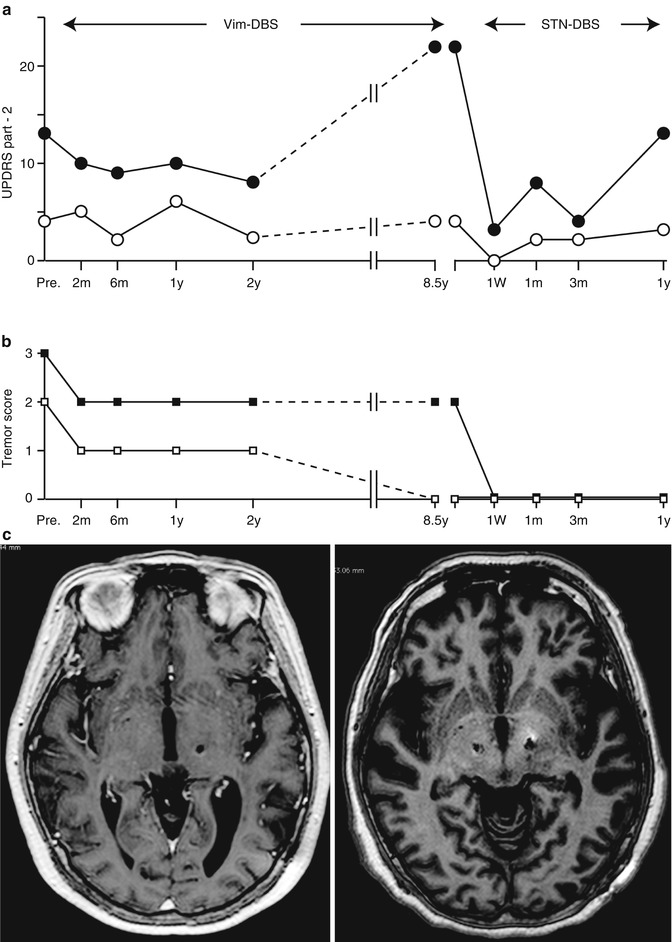
9.12 Vim-DBS or STN DBS?
Tremor-dominant PD is known to exhibit slower disease progression than the akinesia/rigid type of tremor (Zetusky et al. 1985; Vu et al. 2012). On the other hand, it is empirically known that some tremors decrease its severity in exchange for the progression of akinesia or rigidity in the advanced stage of PD. Vim-DBS may have a significant role in improving the quality of life threatened by disabling tremors; however, in PD patients whose tremor was successfully controlled after unilateral and bilateral Vim-DBS over 3 years, 65 % of them had disability due to akinetic-rigid symptoms and levodopa-induced dyskinesias (Pollak et al. 1997, 2002), making the long-term follow up limited to only 15 %. Progression of PD is still inevitable after Vim-DBS, and there was significant increase in dopamine replacement therapy in a long-term follow up (Tarsy et al. 2005). From our operative series, the typical course of disease progression of the PD patient who had unilateral Vim-DBS followed by bilateral STN-DBS was presented in Fig. 9.6. In this case, the activity of daily living (ADL) gradually deteriorated during Vim-DBS because of the progression of akinesia and rigidity although tremor control was stable for over 8 years. Switching to bilateral STN-DBS, the ADL during the off period was significantly improved. Furthermore, tremors completely ceased, which could not be achieved by Vim-DBS. Even if tremor amplitude is very large, the therapeutic targets (Vim, STN and GPi) should be carefully selected in the light of disease progression. For advanced PD patients, STN and GPi are more appropriate targets of DBS to relieve intractable tremors, while also alleviating tremors (Volkmann et al. 1998; Pollak et al. 2002; Krack et al. 2003; Hariz et al. 2008). The effect of STN DBS on rest and postural tremor has been reported to be stable over time (Krack et al. 1998; Rodriguez et al. 1998). STN-DBS affects the network metabolism of cerebello-thalamo-cortical pathways more widely than Vim-DBS (Mure et al. 2011). On the contrary, Vim-DBS could be an appropriate treatment of intractable tremors of PD when the cognitive decline is worried about. Preexisting mild cognitive dysfunction in PD patients was not worsened after Vim-DBS as evaluated by neuropsychological testing (Caparros-Lefebvre et al. 1992), but Vim-DBS exhibited significant improvement on measures of conceptualization, verbal memory, emotional adjustment, and QOL at 1-year follow up (Woods et al. 2001). Tröster et al. (1998) found that Vim-DBS subtly improved semantic verbal fluency but interfered with immediate recall of word lists. It should be argued that Vim-DBS remains a reasonable option for parkinsonian patients with long-standing, non-progressive tremor-dominant variant, especially in the elderly or cognitively impaired cases.