Disorders of Reproduction and Fertility
Andrew G. Herzog
Introduction
Reproductive dysfunction is unusually common among women and men who have epilepsy.27,47,48 It generally manifests as menstrual disorders, hirsutism, and infertility in women48 and diminished libido, impotence, and infertility in men.47 Reproductive dysfunction is often associated with and may be the consequence of abnormal reproductive endocrine function.28,47,48 Both epilepsy and antiepileptic drug (AED) use have been causally implicated.28,38,47,48,50 Epilepsy and antiepileptic drugs can target a number of substrates to impact hormone levels. These include the limbic system, hypothalamus, pituitary, peripheral endocrine glands, liver, and adipose tissue.33,43 Reproductive endocrine disorders, in turn, can lead not only to reproductive dysfunction, but also to exacerbation of epilepsy.33,43 An understanding of these relationships and mechanisms is important to the comprehensive management of women and men with epilepsy.
Prevalence
Hospital-based34,48 and community-based91 studies have shown that menstrual disorders are more common among women with epilepsy than in the general population. Menstrual disorders can be categorized as amenorrhea (no menses for 6 months), oligomenorrhea (cycle intervals >32 days), polymenorrhea (cycle intervals <26 days), abnormal variation in cycle intervals (>4 days), and menometrorrhagia (heavy menses and bleeding between menses40). Cycle intervals between 26 and 32 days, rather than the currently popular broader range of 21 to 35 days, should be considered normal in women with epilepsy because ovulatory rates drop substantially and statistically significantly outside of the 26- to 32-day range, specifically from 75% to <50%.40 Ovulation is considered to be an important criterion in this population because anovulatory cycles are associated with greater seizure frequency.2,42,61 Menstrual disorders, using the foregoing definition, are estimated to occur in one third of women with epilepsy as compared with 12% to 14% of women in the general population.34,40,46 More than one third of cycles in women with localization-related epilepsy (LRE) are anovulatory, as compared to 8% to 10% in controls.12,40,63 There is conflicting evidence as to whether anovulatory cycles are more common with LRE or primary generalized epilepsy (PGE).12,63 Reproductive dysfunction may be the result of reproductive endocrine disorders, especially those that are overrepresented in women with epilepsy.12,34,46,48,63 These include polycystic ovarian syndrome, hypothalamic amenorrhea, functional hyperprolactinemia, and premature menopause.6,27,34,48,56 Women with idiopathic epilepsy are only 37% as likely as unaffected female siblings to become pregnant.86 This finding is not attributable to marital rate or to seizure type, age at onset, or family history of epilepsy.86 In comparison to the general female population, fertility is reduced to 69% to 85% of the expected number of offspring among married women with epilepsy, primarily temporal lobe epilepsy (TLE).14,98 One third of women with epilepsy have sexual dysfunction as determined by standardized questionnaire.35 They may have sexual anxiety as well as deficits in arousal.64 Sexual dysfunction is more common with right-sided rather than left-sided TLE13,35 and is associated with lower serum testosterone levels.35
Sexual dysfunction (diminished sexual interest and/or potency) occurs in about 20% of men with epilepsy as determined by standardized questionnaire.38 Older studies, using mostly structured or unstructured interviews, found higher frequencies ranging from 38% to 71%.33 Abnormal semen analysis, including decreased sperm count, abnormal morphology, and impaired motility, has been reported in >90% of men with epilepsy.8,34,53,92 Semen volume is reduced by 25% and sperm count by 67%.95 Sperm morphologic abnormalities occur in 47%.92 Although AED use is an important factor and differential AED effects on both sexual function38 and semen characteristics54 have been convincingly demonstrated, there is also considerable evidence to suggest that sexual dysfunction, reproductive endocrine disorders, and abnormal semen analysis are also common among untreated men with epilepsy.38,92 Men with idiopathic epilepsy are only 36% as likely as male unaffected siblings to ever father a pregnancy.86 This reduction is associated with LRE, onset of seizures before 20 years of age, and absence of a family history of epilepsy.86 The effect is mitigated by reduced marital rates. Among married men with epilepsy, reproductive disadvantage was confined to those with onset before 10 years of age.86
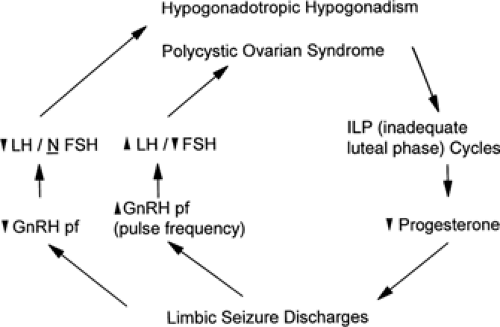 FIGURE 2. Possible mechanisms by which limbic seizure discharges may promote reproductive endocrine disorders and how abnormal reproductive hormone levels may influence epilepsy. This is based on the hypothesis proposed by Herzog et al.28,34,48 that involvement of limbic structures with epileptiform discharges may disrupt normal limbic modulatory influences on hypothalamic gonadotropin-releasing hormone (GnRH) secretion. Altered frequency or amplitude of GnRH may lead to patterns of pituitary luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion that are found in hypogonadotropic hypogonadism (hypothalamic amenorrhea) and polycystic ovarian syndrome. Reproductive endocrine disorders that are associated with partial seizures of temporal lobe origin are characterized by anovulatory cycles and diminished progesterone secretion. An elevated serum estrogen/progesterone ratio may promote the development of seizure discharges in the brain. |
Mechanisms in Women
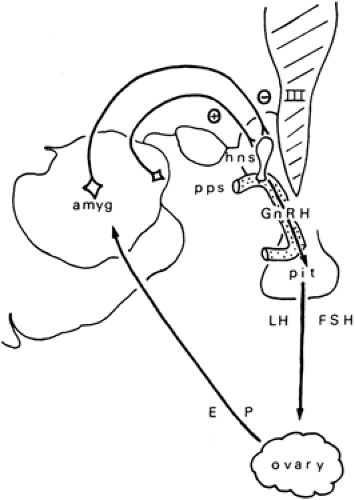
Reproductive dysfunction is often associated with and may be the consequence of abnormal reproductive endocrine function.28,34,48 This, in turn, may result from altered neuroendocrine regulation due to epilepsy itself.7,34,48 Epilepsy may alter the hypothalamopituitary regulation of gonadotropin secretion (Figs. 1 and 2) or may have direct neurally mediated dystrophic effects on the ovaries, mediated by the autonomic nervous system.25 The neurotrophic effects have been a much-neglected investigational topic but may warrant attention in an era in which vagal nerve stimulators are in common use. AEDs may alter peripheral steroid and binding protein synthesis as well as hormonal metabolism53,62 and may produce direct gonadal toxicity.78,94
The most common reproductive endocrine disorder in women in general and in the epilepsy population in particular is polycystic ovarian syndrome (PCOS).6,46,48 Its overrepresentation in women with epilepsy6,27,34,46,48,56 (10%–20% in women with epilepsy vs. 5%–6% in general population studies) may be of considerable significance because in the general population, PCOS is associated with a higher prevalence of
migraine, emotional disorders, diabetes, cardiovascular disease, and female cancers.46 Hypothalamic amenorrhea, functional hyperprolactinemia, and premature menopause have also been found to be overrepresented.6,27,34,46,48,56 In an investigation of 50 consecutive women with clinical and electroencephalographic features of TLE, 28 (56%) had amenorrhea, oligomenorrhea, or abnormally long or short menstrual cycle intervals.48 Nineteen of the 28 women with epilepsy and menstrual disorders (68%, 38% overall) had readily identifiable reproductive endocrine disorders: PCOS in 10, hypothalamic amenorrhea in 6 (12%), premature menopause in 2 (4%), and functional hyperprolactinemia in 1 (2%). The numbers of women with clinical and endocrine features of PCOS (20%) and of hypogonadotropic hypogonadism (HH) (12%) were significantly greater than the estimated frequencies (5% for PCOS and 1.5% for HH) in the general female population. The data showed no significant relationship overall between the occurrence of menstrual disorders and the use of AEDs (53% among users vs. 60% among nonusers). Moreover, PCOS was more common among the untreated (30%) than the treated (13%) women with epilepsy, although since then, a notable relationship between the use of valproate, not yet in common use at the time of the investigation, and PCOS51 has been demonstrated.
migraine, emotional disorders, diabetes, cardiovascular disease, and female cancers.46 Hypothalamic amenorrhea, functional hyperprolactinemia, and premature menopause have also been found to be overrepresented.6,27,34,46,48,56 In an investigation of 50 consecutive women with clinical and electroencephalographic features of TLE, 28 (56%) had amenorrhea, oligomenorrhea, or abnormally long or short menstrual cycle intervals.48 Nineteen of the 28 women with epilepsy and menstrual disorders (68%, 38% overall) had readily identifiable reproductive endocrine disorders: PCOS in 10, hypothalamic amenorrhea in 6 (12%), premature menopause in 2 (4%), and functional hyperprolactinemia in 1 (2%). The numbers of women with clinical and endocrine features of PCOS (20%) and of hypogonadotropic hypogonadism (HH) (12%) were significantly greater than the estimated frequencies (5% for PCOS and 1.5% for HH) in the general female population. The data showed no significant relationship overall between the occurrence of menstrual disorders and the use of AEDs (53% among users vs. 60% among nonusers). Moreover, PCOS was more common among the untreated (30%) than the treated (13%) women with epilepsy, although since then, a notable relationship between the use of valproate, not yet in common use at the time of the investigation, and PCOS51 has been demonstrated.
PCOS is unlikely to represent a single nosologic entity but rather the failure of the ovarian follicle to complete normal maturation during the menstrual cycle or a series of cycles.46 PCOS may represent a common endpoint for a number of pathophysiologic conditions, some of which may be attributable to epilepsy itself6,34,48 or AED use.50,63 The partially developed follicle is secretory but is deficient in aromatase, the enzyme that converts testosterone to estrogen, and, having failed to release its ovum, is retained in the ovary in the form of a cyst.46 (The persistent occurrence of such cycles results in hyperandrogenic chronic anovulation, which is currently the simplest and perhaps the most utilitarian definition of PCOS.46,59)
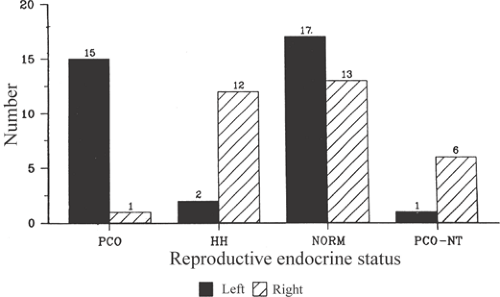
There are findings that implicate epilepsy itself in the pathogenesis of PCOS. Herzog et al.29,34,46,48 reported an association between particular reproductive endocrine disorders and the laterality of unilateral temporolimbic epilepsy. Specifically, left laterality has been associated with PCOS, whereas right laterality has been associated with hypothalamic amenorrhea. Nontemporal foci may have different laterality relationships (Fig. 3).29 Herzog et al.34 also evaluated reproductive endocrine function in women with unilateral temporolimbic epilepsy and normal controls to assess the effects of epilepsy, epilepsy laterality, and AED use on the cerebral regulation of hormonal secretion. The findings indicate that reproductive endocrine function differs between women with epilepsy and normal controls. Significant differences exist at all levels of the reproductive neuroendocrine axis, that is, hypothalamus, pituitary, and peripheral gland. Differences show significant relationships to epilepsy itself as well as to medication use. Sex hormone changes occur in a stochastic manner such that the laterality of unilateral temporolimbic discharges is associated with predictable directional changes in hormonal secretion at all levels of the reproductive neuroendocrine axis. These directional changes are consistent with the finding that different reproductive disorders may develop in relation to left- and right-sided temporolimbic epilepsy. Some hormonal changes can show close temporal relationship to the occurrence of interictal epileptiform discharges, and these vary in relation to the laterality of the discharges. Antiepileptic drugs differ in their effects on reproductive hormone levels. There are notable
differences between enzyme-inducing and noninducing drugs, with the former being associated with lower serum levels of some ovarian and adrenal steroids, such as estradiol, testosterone, and dehydroepiandrosterone sulfate. Menstrual disorders are more common among women with interictal discharges as well as among women with abnormal hormonal findings.
differences between enzyme-inducing and noninducing drugs, with the former being associated with lower serum levels of some ovarian and adrenal steroids, such as estradiol, testosterone, and dehydroepiandrosterone sulfate. Menstrual disorders are more common among women with interictal discharges as well as among women with abnormal hormonal findings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree