Chapter 46 Drugs That Disturb Sleep and Wakefulness
Abstract
Research on the neural mechanisms involved in sleep–wake regulation suggest that sleep–wake state is controlled by a complex interaction between wakefulness-promoting and sleep-promoting nuclei in the hypothalamus and brainstem.1–3 Wake-promoting neurons include orexinergic and histaminergic nuclei in the hypothalamus, cholinergic nuclei in the brainstem, adrenergic nuclei in the locus coeruleus, serotonergic nuclei in the raphe nuclei, and dopaminergic nuclei in the midbrain ventral tegmental area. Sleep is promoted by nuclei in the basal forebrain, ventrolateral preoptic area, and anterior hypothalamus through the inhibitory neurotransmitters gamma-aminobutyric acid (GABA) and galanin. Adenosine, which has been proposed to be involved in homeostatic regulation of sleep, may promote sleep through anticholinergic activity in the basal forebrain and brainstem. Drugs can cause sedation by multiple mechanisms, either by increasing the activity of the sleep-promoting system through GABA enhancement (e.g., benzodiazepine receptor agonists, ethanol) or by inhibiting the wake-promoting system by antagonism of central histamine type 1 (H1) receptors (e.g., first-generation antihistamines, tricyclic antidepressants), alpha-adrenergic type 1 (alpha1) receptors (e.g., clonidine, certain antidepressant and antipsychotic medications), muscarinic cholinergic receptors (e.g., some antidepressants), or serotonin 5-HT2A receptors (e.g., eplivanserin, under development for insomnia). Similarly, drugs can disrupt sleep through effects on either the sleep-promoting system (e.g., inhibition of GABA by caffeine) or the wake-promoting system (e.g., stimulation of dopamine release or blockade of dopamine reuptake by amphetamines). Drugs may also affect homeostatic and circadian processes involved in sleep–wake regulation. Effects on neurotransmitters and neuronal systems involved in the generation of slow-wave sleep (SWS) and rapid eye movement (REM) sleep can affect sleep architecture.
This chapter reviews drugs that are used for common medical and psychiatric conditions and that have unintended effects on sleep or wakefulness. Drugs used as hypnotics are reviewed in Chapters 42 and 43. Stimulants, including caffeine, and drugs of abuse, including alcohol and nicotine, are discussed in Chapters 44 and 45.
Psychotherapeutic Drugs
Antidepressants
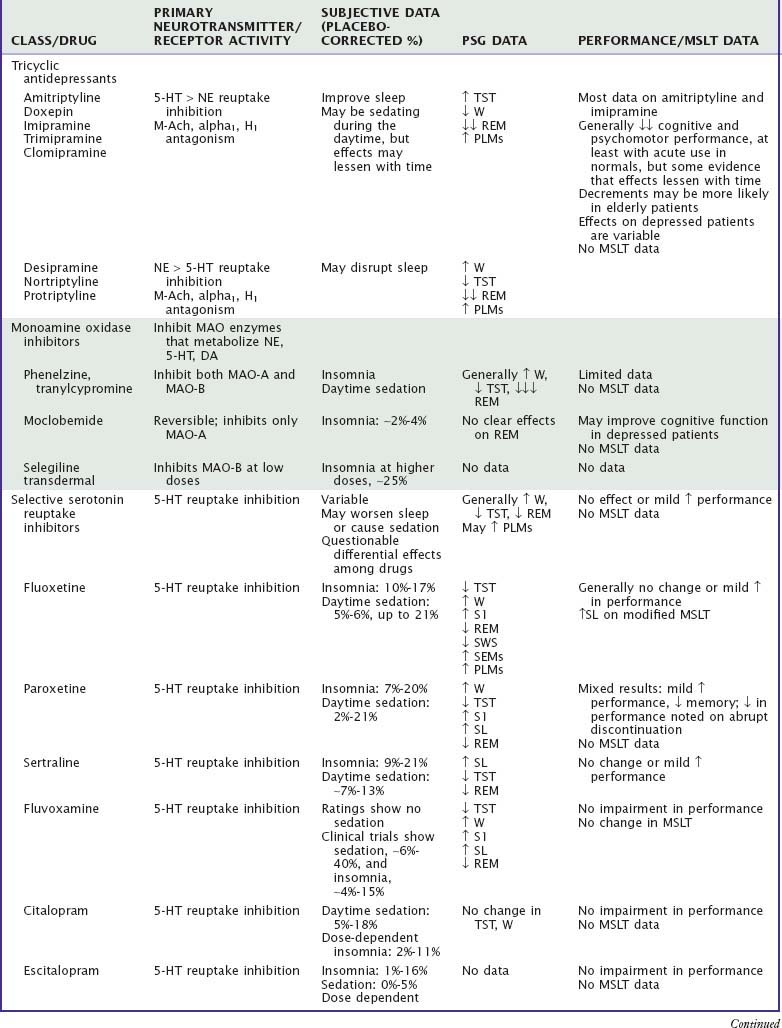
Table 46-1 summarizes the effects of antidepressant drugs on sleep and waking behavior. Sedating antidepressants used as hypnotics are covered more fully in Chapter 43.
Antidepressant drugs can improve or disturb sleep as well as affect waking function. Evaluation of the effects of these drugs on sleep and wakefulness is complicated by the fact that many individuals with depression have disturbed sleep4 as well as daytime complaints, such as fatigue, sleepiness, somatic complaints, and decreased cognitive and psychomotor functioning.5–7 In addition, polysomnographic (PSG) evidence of disturbed nocturnal sleep does not always correlate with the subjective reports of patients and is not related to the efficacy of these drugs in the treatment of depression.
Tricyclic Antidepressants
Tricyclic antidepressants (TCAs) differ from one another in their relative effects in blocking serotonin versus norepinephrine reuptake as well as in the degree of antagonism of muscarinic cholinergic receptors and H1 histamine receptors.8 The more sedating TCAs tend to be more anticholinergic (amitriptyline) and more antihistaminergic (doxepin, trimipramine) but also exhibit proportionately greater inhibition of serotonin reuptake than of norepinephrine reuptake. In general, these drugs decrease sleep latency, increase total sleep time (TST), and decrease REM sleep while increasing phasic eye movements during REM.9,10 TCAs that are more adrenergic (e.g., desipramine, nortriptyline) may decrease TST and increase awakenings.11 TCAs may increase periodic limb movements and symptoms of restless legs syndrome.10 Cognitive performance and psychomotor performance are impaired with acute use in normals, but there is some evidence that these effects lessen with time.12 Low-dose doxepin has been approved as a hypnotic.12a
Monoamine Oxidase Inhibitors
Monoamine oxidase inhibitors (MAOIs) inhibit the action of MAO enzymes, resulting in increased concentrations of serotonin, norepinephrine, and dopamine. The classic MAOIs (e.g., isocarboxazid, phenelzine, tranylcypromine) irreversibly inhibit both MAO-A and MAO-B enzymes. Insomnia and daytime sedation are commonly reported side effects (up to 62% and 42% of patients, respectively),13 but there are no placebo-controlled studies. The most impressive PSG finding is a marked decrease in REM sleep, including almost complete abolishment of REM sleep.14 TST is also decreased. Although multiple sleep latency test (MSLT) studies are lacking, actigraphic monitoring in a small group of patients confirmed periods of decreased daytime activity coincident with reported episodes of napping, possibly associated with poor nighttime sleep. Cognitive performance and psychomotor performance do not appear to be influenced by the classic MAOIs, but data are limited.12,15 The newer MAOIs reversibly or selectively inhibit the MAO-A enzyme, resulting in fewer severe adverse effects. Drugs of this type include moclobemide (not available in the United States) and selegiline. Subjective and PSG data suggest that insomnia is more likely with higher doses.16,17 Moclobemide may enhance cognitive function in depressed outpatients.15
Selective Serotonin Reuptake Inhibitors
Selective serotonin reuptake inhibitors (SSRIs) selectively and potently inhibit the reuptake of serotonin, but they also exhibit norepinephrine and dopamine reuptake inhibition, muscarinic cholinergic antagonism, inhibition of various cytochrome P-450 enzymes, and other actions. In addition, numerous serotonin receptor subtypes mediate a variety of actions. Individual SSRIs also differ in their effects on serotonin receptor subtypes as well as in their actions at other receptors, which may explain why SSRIs may be associated with both insomnia9,18 and daytime sedation.5,9,19
Polysomnographic studies of SSRIs generally indicate disruption of sleep continuity and suppression of REM sleep.11,20 Fluoxetine, which has been studied most extensively, decreases TST and increases wake time and stage 1 sleep both in normal subjects during single-night studies with doses of 20 to 60 mg21 and in depressed patients with doses of 20 to 80 mg for up to 1 year.22 Fluoxetine has been associated with prominent slow eye movements in non-REM sleep.23 SSRIs are also associated with increased frequency of periodic limb movements during sleep, restless legs syndrome,24 and REM sleep without atonia.25 Paroxetine (15 to 30 mg) decreases TST and increases awakenings in normal subjects with 1- to 2-day dosing.26 There also is evidence of increased awakenings and sleep fragmentation after 5 weeks of paroxetine treatment in depressed inpatients.27 Fluvoxamine has had similar effects on the sleep architecture of depressed patients.28 Citalopram produced the typical decrease in REM sleep but no changes in sleep latency or TST during 5 weeks of treatment in one study of depressed patients.29
SSRIs usually do not negatively affect daytime performance or cognitive functioning and may actually improve functioning in some patients, but data are limited. One placebo-controlled study reported memory impairment with paroxetine but improvement in a verbal task with sertraline.30 A single nighttime dose of fluvoxamine in healthy subjects showed increased daytime sleep latencies compared with dothiepin but no change compared with placebo in a modified MSLT study.31
Serotonin and Norepinephrine Reuptake Inhibitors
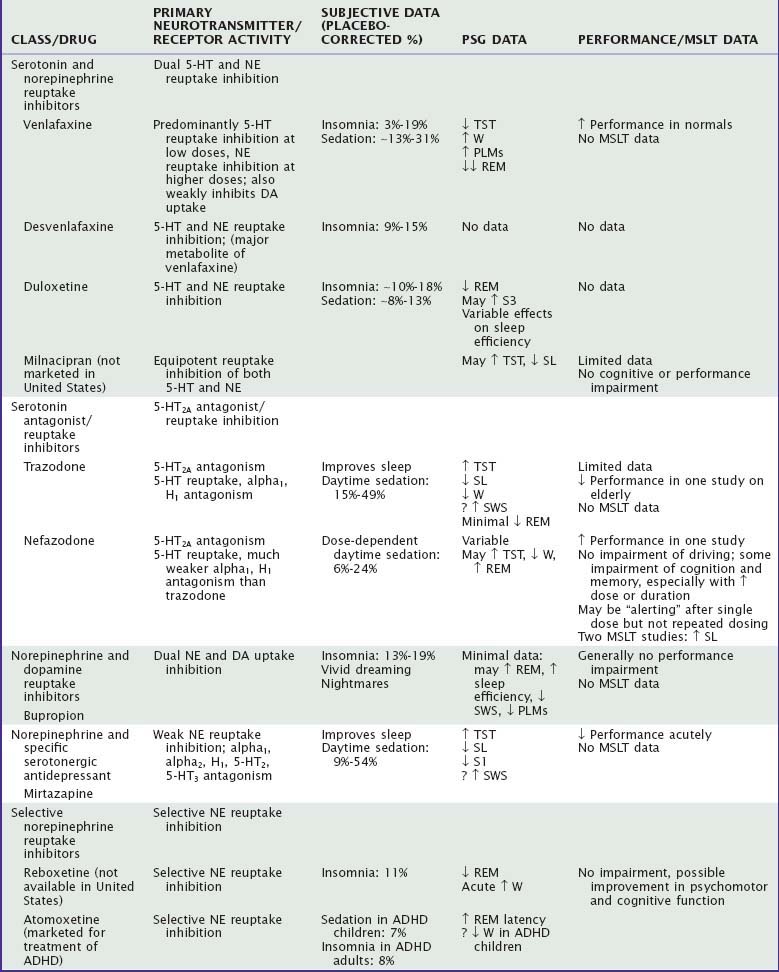
Serotonin and norepinephrine reuptake inhibitors (SNRIs), which include venlafaxine, desvenlafaxine, duloxetine, and milnacipran (not available in the United States), inhibit reuptake of both norepinephrine and serotonin. In addition to treatment of both anxiety and depression, duloxetine has been approved for treatment of neuropathic pain and has been evaluated for treatment of fibromyalgia.32 Both insomnia and somnolence have been reported by patients taking these drugs.33–35 In normal subjects, 75 to 150 mg of venlafaxine produced increased wake and stage 1 sleep; in addition, in six of the eight subjects, frequent periodic limb movements (more than 25 per hour) were noted.36 In depressed inpatients treated for 1 month, venlafaxine (maximum dose, 225 mg/day) increased PSG-recorded wake after sleep onset.37 In one study of normal subjects,38 sleep efficiency was decreased with duloxetine 60 mg twice daily but increased by duloxetine 80 mg/day. In a study of depressed patients,39 duloxetine 60 to 90 mg/day had no effect on sleep continuity by increased stage 3 sleep. REM sleep was suppressed in both studies. There are no studies that objectively evaluate daytime sleepiness and alertness. Single doses of venlafaxine improved performance and cognitive function in healthy subjects, particularly 6 to 8 hours after dosing.40
Serotonin Antagonist and Reuptake Inhibitors
Trazodone and nefazodone are more fully covered in Chapter 43. Whereas both drugs block serotonin reuptake and inhibit 5-HT2 receptors, trazodone also shows histamine H1 antagonism as well as adrenergic alpha1 and alpha2 antagonism.41 Drowsiness is the most commonly reported side effect of trazodone.33 Because of its sedation, trazodone is currently more likely to be used as a hypnotic than as an antidepressant. PSG studies indicate that trazodone decreases sleep latency, may improve sleep continuity, increases SWS, but does not affect REM sleep. Nefazodone may increase TST but has no consistent effect on sleep latency, SWS, or REM sleep.9,42 One study in normal individuals demonstrated an increase in MSLT mean latency (i.e., decreased sleepiness) after 16 days of nefazodone treatment.43 Trazodone impairs performance in healthy individuals,44 but data on depressed individuals are inconclusive. Cognitive and performance studies of nefazodone in general show no impairment or even slight improvement in psychomotor performance and memory in middle-aged and elderly healthy subjects.45,46
Norepinephrine and Dopamine Reuptake Inhibitors
Bupropion, which inhibits the uptake of dopamine and norepinephrine, is associated with insomnia in 5% to 19% of patients in clinical trials.47 In a PSG study of seven depressed patients, after 4 weeks of treatment, bupropion did not affect sleep latency or TST but did decrease REM latency and increase REM sleep percentage.46 In a study of depressed patients with periodic limb movements, sustained-release bupropion decreased periodic limb movements, possibly because of its effects on dopamine reuptake.48 Bupropion is not usually associated with cognitive or psychomotor performance impairment.44,49
Norepinephrine and Specific Serotonin Antagonists
Mirtazapine, sometimes used as a hypnotic, is described more fully in Chapter 43. Mirtazapine disinhibits both serotonin and norepinephrine through blockade of alpha2 receptors.50 It is a potent antagonist of histamine H1 and 5-HT2 receptors, which probably accounts for its highly sedating activity. PSG studies indicate improvements in sleep latency and sleep continuity.51 One study suggests that restless legs syndrome symptoms develop or worsen in up to 28% of patients.52 Mirtazapine impairs driving performance and attention acutely in normals.53
Selective Norepinephrine Reuptake Inhibitors
Reboxetine, not currently available in the United States, is a selective norepinephrine reuptake inhibitor. Clinical trials indicate an incidence of insomnia of approximately 10%.54 PSG studies in depressed patients showed acute increases in wake and persistent suppression of REM sleep.55 In healthy normals, reboxetine was subjectively sedating but did not impair cognitive function.56
Atomoxetine, also a selective norepinephrine reuptake inhibitor but not developed as an antidepressant, is approved for treatment of attention-deficit/hyperactivity disorder (ADHD). Clinical trials indicate somnolence in ADHD children (7% incidence) but insomnia in ADHD adults (8% incidence). A PSG study in ADHD children reported that atomoxetine increased REM latency but did not adversely affect sleep latency (unlike methylphenidate).57
Miscellaneous Antidepressants
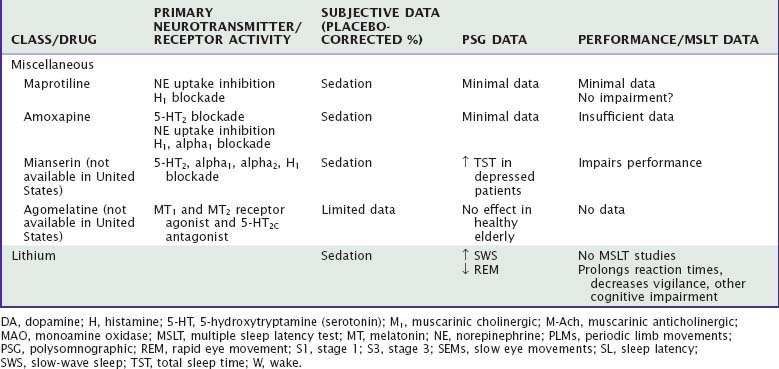
Amoxapine, a dibenzoxazepine tricyclic, and maprotiline and mianserin (not available in the United States), both tetracyclics, have sedative profiles similar to those of the TCAs, probably because of their high affinity for H1 receptors.58 Amoxapine and maprotiline are not commonly used because of other serious side effects, namely, seizures in the case of maprotiline and dopamine-blocking side effects with amoxapine. There are no studies objectively measuring sleepiness. Minimal data suggest little cognitive impairment with maprotiline.15 In healthy individuals, mianserin impaired driving and tracking performance as well as reaction time and other psychomotor measures.12,44 Agomelatine, recently approved for treatment of depression in Europe, is a potent agonist at both melatonin MT1 and MT2 receptors as well as an antagonist at serotonergic 5-HT2C receptors. Limited non–placebo-controlled PSG data in depressed patients showed increases in sleep efficiency and SWS without effects on REM sleep compared with baseline.59
Lithium
Lithium, which is used primarily in the treatment of manic-depressive illness, is subjectively associated with improved nocturnal sleep and increased daytime sleepiness, at least initially.60 Sleep disturbance is a prominent feature of mania and similar polysomnographically to that observed in major depression.61 In healthy volunteers, lithium increases SWS and decreases REM sleep62; it produces cognitive and psychomotor deficits, including prolonged reaction times, decreased vigilance, and impairment of semantic reasoning.61 Similar deficits have been shown in psychiatric patients taking lithium for periods ranging from 2 weeks to longer than 3 months,63 although it is difficult to determine whether the deficits seen in the patient population are caused by the medication or the psychiatric illness. Degree of cognitive deficit increases with age, severity of disease, and lithium concentration.
Antipsychotic Drugs
Table 46-2 summarizes the effects of antipsychotic drugs on sleep and waking behavior. Sedating drugs used as hypnotics (olanzapine, quetiapine) are covered more fully in Chapter 43.
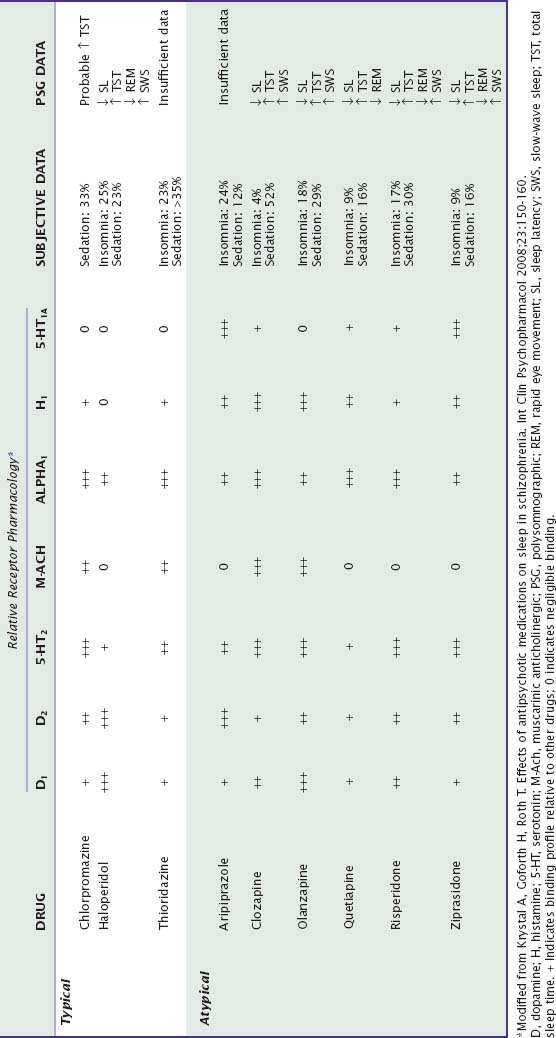
Antipsychotic drugs have complex pharmacologic profiles. Their antipsychotic effects are thought to be mediated primarily by antagonism of dopamine D2 receptors in the mesolimbic dopamine pathway.64 These drugs also block dopamine receptors in other neural pathways, leading to unwanted effects such as anhedonia, extrapyramidal symptoms, and hyperprolactinemia, which are commonly seen with the older or “typical” antipsychotics (e.g., chlorpromazine, haloperidol, thioridazine). These effects are decreased with the newer or “atypical” antipsychotic drugs, which in addition to blockade of dopamine receptors also antagonize serotonin receptors (especially 5-HT2A), have the ability to dissociate from D2 receptors, may have partial agonist activity at D2 receptors, or may act as partial agonists at serotonin 5-HT1A receptors.50 These drugs differ in their specificity for dopamine D2 versus D1 receptors and also differ in the degree to which they block muscarinic cholinergic, histamine, and alpha-adrenergic receptors (see Table 46-2).65 Sedation is more likely in drugs with relatively more potent antagonism of histamine, alpha-adrenergic, or 5-HT2 receptors compared with antagonism of dopamine receptors.66
Patients with schizophrenia commonly have insomnia and circadian rhythm disturbances as well as cognitive impairment, complicating evaluation of the effects of antipsychotic medications. Sedation is a common side effect, but insomnia has also been reported. Among the older drugs, chlorpromazine and thioridazine appear to be more sedating than haloperidol. Clozapine is even more sedating than these drugs (with transient sedation reported by 54% of patients, persistent sedation by 46%, and sedation requiring drug discontinuation by 24%)66; aripiprazole is the least sedating. Whereas ziprasidone and quetiapine would be expected to be sedating given their pharmacologic profiles, they appear less sedating than other drugs, possibly because of their short half-lives. Insomnia occurs at variable rates with these drugs. Although the mechanism is not clear, options include 5-HT1A receptor agonism and restless legs symptoms secondary to dopaminergic antagonism.64 Restless legs syndrome symptoms have been reported as case reports for olanzapine, risperidone, quetiapine, and clozapine.67
There are few double-blind placebo-controlled PSG studies of antipsychotics in healthy normals and none in schizophrenia patients. Small-sample controlled studies indicate that olanzapine, quetiapine, and ziprasidone decrease sleep latency and improve sleep continuity.64,67,68 Uncontrolled studies suggest that clozapine, haloperidol, and risperidone also improve sleep.69 REM suppression is variable, whereas increased SWS is typical, except for quetiapine.64,67
There are no MSLT or other studies that objectively evaluate daytime sleepiness.
Although antipsychotics cause cognitive impairment in healthy subjects,70 these drugs may have no negative effects in patient populations.71 Some of the newer drugs, in particular, appear to be more likely to improve cognitive function in patients despite significant sedation.65
Anxiolytic Drugs
The primary drugs used in the treatment of anxiety disorders include antidepressants (particularly SSRIs and SNRIs), benzodiazepines, and buspirone, a 5-HT1A receptor partial agonist. Antiepileptics and atypical antipsychotics are also sometimes used. Prazosin, an antihypertensive with alpha1-adrenergic antagonism, has been used to treat nightmares in posttraumatic stress disorder.72 Antidepressants, antiepileptics, and antipsychotics are covered elsewhere in this chapter. Benzodiazepines approved for treatment of anxiety disorders (e.g., alprazolam, clonazepam, diazepam, lorazepam) have pharmacologic profiles similar to those of benzodiazepines used to treat insomnia and thus similar side effects. Given that these drugs enhance GABA at the GABAA receptor, their most common side effect is sedation.60 In nonanxious subjects, daytime administration of alprazolam and diazepam produced decreased sleep latencies as measured by MSLT on both day 1 and day 7 of treatment, with alprazolam producing greater sleepiness than diazepam on the first day of treatment.73 Performance impairment, including impairment of actual driving performance,74 is common with benzodiazepines in studies of normal subjects and patient groups for treatment periods of up to 3 weeks, particularly at higher doses. Well-controlled studies are needed to determine whether longer term use of benzodiazepine anxiolytics results in tolerance to these performance-impairing effects and whether there are differential effects between younger and older individuals.
Buspirone does not have the hypnotic, anticonvulsant, and muscle relaxant properties of the benzodiazepines. The anxiolytic efficacy of buspirone is similar to that of the benzodiazepines, but its onset of action is much slower, requiring up to 3 to 4 weeks.75 Buspirone appears to act primarily as a 5-HT1A partial agonist but also has effects on D2 receptors.76 It has no affinity for the benzodiazepine receptors and does not affect GABA binding. In clinical studies of anxious patients, buspirone was comparable to placebo in the frequency of subjective reports of sedation.77 In a study of 12 patients with chronic insomnia, alertness as measured by MSLT was not impaired by buspirone 20 mg/day in divided doses during a 3-day period.78 Compared with benzodiazepine anxiolytics, buspirone appears to have few negative effects on psychomotor, cognitive, or driving performance in healthy volunteers receiving short-term treatment or in patients treated for up to 4 weeks.78,79
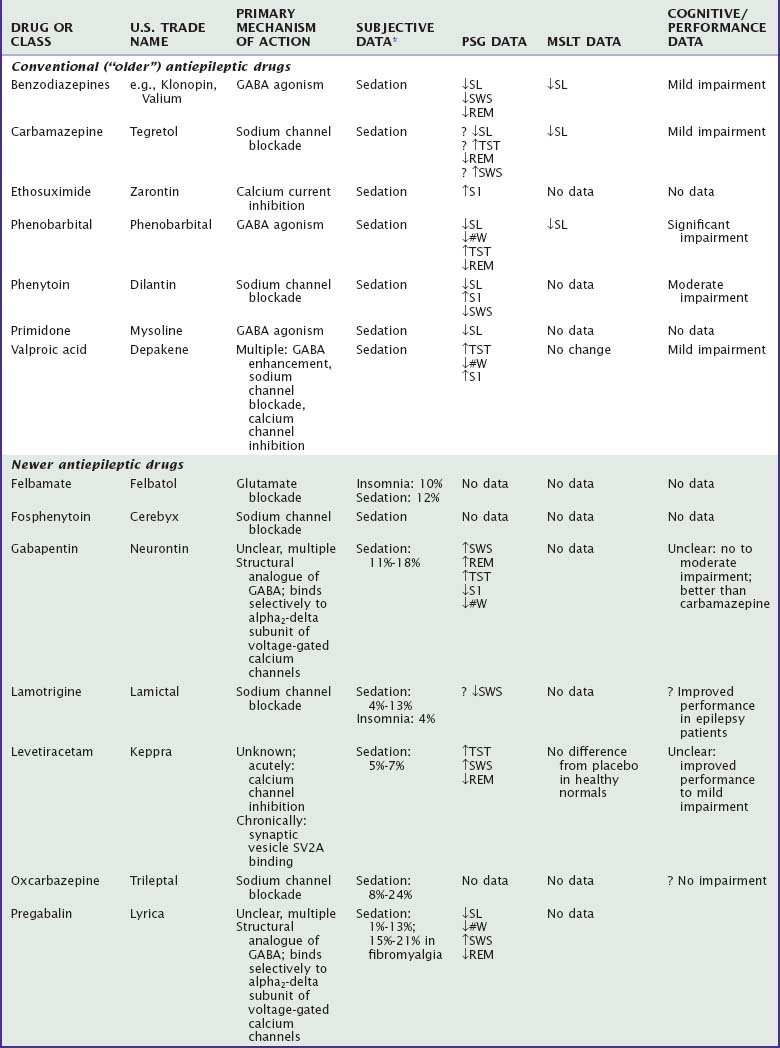
Antiepileptic Drugs
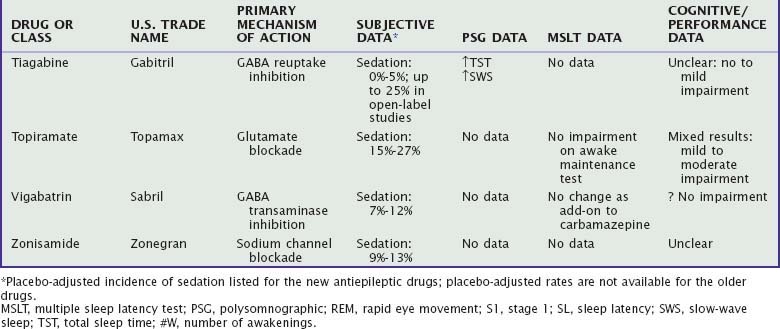
There are many antiepileptic drugs available for use, generally classified somewhat arbitrarily as “older” or “newer.”80 The older or “conventional “ drugs include benzodiazepines, carbamazepine, ethosuximide, phenobarbital, phenytoin, primidone, and valproate. Findings regarding the effects of these medications on sleep and waking are summarized in Table 46-3. Mechanisms of action include sodium channel blockade, effects on GABA (agonism, reuptake inhibition, transaminase inhibition), and glutamate blockade as well as unknown mechanisms (e.g., levetiracetam).81,82 Some of the newer drugs have a variety of mechanisms of action, which has led to their evaluation and use in other neurologic and psychiatric conditions, such as neuropathic pain, fibromyalgia, migraine, essential tremor, anxiety, schizophrenia, and bipolar disorder.83 Although developed as antiepileptic drugs, gabapentin and pregabalin are used more frequently in the treatment of neuropathic pain and fibromyalgia and, along with tiagabine, have been evaluated for insomnia.
Subjectively, sedation is one of the most common adverse effects of the older antiepileptic drugs, particularly phenobarbital.82,84 Among the newer antiepileptic drugs, the incidence of reported sedation in placebo-controlled clinical trials is 5% to 15% for gabapentin, lamotrigine, levetiracetam, vigabatrin, and zonisamide and 15% to 27% for topiramate, and it is generally more prevalent during the initial treatment.85,86 The incidence of sedation with tiagabine was no different from that for placebo in placebo-controlled studies, but it was 25% in open-label, long-term studies.87 Both sedation and insomnia have been reported with felbamate. Approximately 15% to 25% of patients with neuropathic pain or fibromyalgia treated with gabapentin or pregabalin reported somnolence.88,89
Polysomnographic studies of established antiepileptic drugs in general show that these drugs produce shorter sleep latency and increase TST.82 The newer drugs show variable effects on sleep latency and sleep continuity (see Table 46-3). However, levetiracetam, gabapentin, pregabalin, and tiagabine increase SWS, whereas lamotrigine decreases SWS.90–93
Placebo-controlled studies in healthy normals indicate no decrement in MSLT latencies with levetiracetam93 and no increase in drowsiness on a 6-minute “awake maintenance test” with either gabapentin or topiramate, although gabapentin produced electroencephalographic slowing.94 In studies without placebo control, patients treated with phenobarbital95 or carbamazepine96 had lower mean MSLT latencies, and patients receiving carbamazepine, phenytoin, phenobarbital, or valproate demonstrated increased drowsiness on the awake maintenance test compared with healthy control subjects and untreated patients with epilepsy.85 Drug-naive patients with partial epilepsy receiving topiramate showed no change in MSLT latencies compared with healthy control subjects either at baseline or 2 months later.97
Cognitive impairment appears to be more common with phenobarbital than with other drugs, more common when multiple medications are used, and more common in children than in adults. Phenobarbital and phenytoin have most frequently been associated with significantly impaired neuropsychological function, particularly in the areas of short-term memory, concentration, and attention.95,98,99 Carbamazepine and valproate appear to be mildly impairing, whereas the newer antiepileptic drugs appear to have few negative cognitive effects, with the exception of topiramate.94,98,100,101 However, well-controlled studies are rare, and methodological problems include subject composition, choice of neuropsychological test, lack of placebo control, and sample size.100 As a number of these drugs are being increasingly used in nonepilepsy disorders,83 the need for carefully controlled studies evaluating the effects of these drugs on daytime function is increased.
Antiparkinsonian Drugs
See Chapter 87 for additional information on Parkinson’s disease and its treatment. Dopaminergic drugs used in the treatment of restless legs syndrome are further discussed in Chapter 90.
Sleep complaints are extremely common in Parkinson’s disease and include insomnia, parasomnias, and daytime sleepiness.102 These complaints, which tend to worsen with disease progression, may be the result of abnormalities in sleep–wake regulation caused by the disease, accompanying symptoms such as nocturnal motor disturbance, other sleep disorders such as sleep apnea or periodic limb movements, concurrent medical or psychiatric illness, or the medications used for treatment. PSG studies of Parkinson’s patients show increased sleep fragmentation103 and decreased REM sleep and SWS102 along with increased incidence of REM behavior disorder.104 Periodic limb movements and sleep apnea are also common. MSLT studies indicate a high prevalence of excessive daytime sleepiness.105 Dopamine replacement is the primary treatment of Parkinson’s disease. Because sleep is significantly disrupted in Parkinson’s disease, it is difficult to determine whether changes in sleep and waking behavior after drug administration are due to direct effects of drug or effects of drug on disease.
The principal drugs used to treat Parkinson’s disease are levodopa/carbidopa and dopamine agonists, which include ergot agonists (apomorphine, bromocriptine, cabergoline, lisuride, piribedil, and pergolide) and nonergot agonists (pramipexole, ropinirole, and rotigotine). Amantadine is less commonly used. Adjunctive treatments include catechol-O-methyltransferase inhibitors (e.g., entacapone, tolcapone), which prolong the duration of the effect of levodopa106; anticholinergics (e.g., hyoscyamine, benztropine); and selegiline,107 an MAO-B inhibitor that preferentially oxidizes dopamine and phenylethylamine and is metabolized to methamphetamine and amphetamine.
Dopamine agonists differ somewhat in their selectivity for dopamine receptor subtypes. There are five subtypes of dopamine receptors divided into two major classes, D1 (D1 and D5 subtypes) and D2 (D2, D3, D4 subtypes).108 The nonergot drugs have higher selectivity than the ergot agonists.109 Pergolide and apomorphine have both D1 and D2 agonist activity; pramipexole, ropinirole, and rotigotine are D2 selective. All three have higher specificity for the D3 receptor subtype than for the D2 subtype; however, pramipexole exhibits the highest specificity. Ergot-derived drugs also demonstrate 5-HT2A and 5-HT2B agonism. Long-term use of the ergot agonists cabergoline and pergolide has been associated with valvular heart disease, possibly associated with 5-HT2B (and perhaps 5-HT2A) agonism.109
There are some data to suggest that low doses of dopaminergic medications tend to improve sleep, whereas higher doses are likely to disrupt sleep.102,109 PSG studies have shown mixed results, including both increased and decreased REM sleep and decreased SWS. Nightmares and visual hallucinations may be increased. Improvement in parkinsonian symptoms may independently improve sleep.
Although dopaminergic agonists have been associated with increases in daytime sleepiness, including sudden “sleep attacks,”110 there is controversy as to whether the sleepiness is related to the pathologic process of Parkinson’s disease or the specific drugs. Initial reports suggested that unintentional sleep episodes were related specifically to the nonergot dopamine agonists. However, subsequent data have indicated no difference in sleepiness or unintentional sleep episodes between ergot and nonergot agonists.111,112 MSLT studies indicate no differences in sleepiness among various dopamine agonists including pramipexole, ropinirole, bromocriptine, or pergolide, taken alone or in combination with levodopa113,114 or compared with levodopa alone.115 Several studies suggest that higher doses of dopaminergic drugs are more likely to be associated with increased sleepiness.116,117 However, higher doses are also associated with more advanced disease.
Cognitive and motor deficits are common in Parkinson’s disease. Anticholinergic drugs have been demonstrated to produce worsening of cognitive function, primarily in the areas of memory function.118 In healthy subjects, ropinirole resulted in improved fine motor activity and reaction time,119 whereas pramipexole increased subjective sedation and impaired cognitive performance,120 and pergolide impaired delayed response tasks but not memory or executive function.121 In patients with mild Parkinson’s disease, pramipexole worsened verbal fluency, whereas pergolide and L-dopa did not.122 Evaluation of the effects of these drugs on cognitive function is complicated by the frequent presence of behavioral symptoms that may affect performance.
Dopaminergic medications are also used in the treatment of restless legs syndrome. Somnolence and fatigue have been reported with pramipexole and ropinirole.123 PSG data on pramipexole, ropinirole, and cabergoline generally show improvements in sleep in this population.124–126 However, these improvements are likely to be the result of treatment of symptoms rather than sedating effect of drug. In a single MSLT study in healthy normals, ropinirole decreased mean MSLT latency.127
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree