7 Electrical events
Structure of the Plasma Membrane
In common with cells elsewhere, the plasma membrane of neurons is a double layer (bilayer) of phospholipids made up of phosphate heads facing the aqueous media of the extracellular and intracellular spaces, and paired lipid tails forming a fatty membrane in between (Figure 7.1). The phosphate layer is water-soluble (hydrophilic, or polar) and the double lipid layer is water-insoluble (hydrophobic, or non-polar).
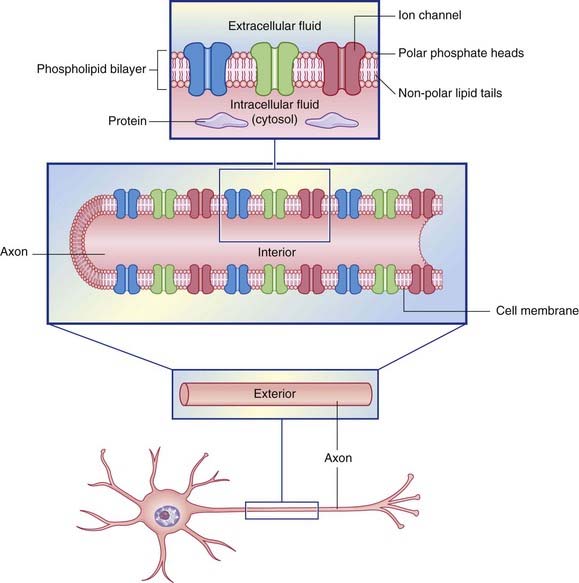
Figure 7.1 Structure of the neuronal cell membrane. The only membrane proteins shown here are ion channels.
Ion channels
Several channel categories are recognized, of which the first three are of immediate relevance.
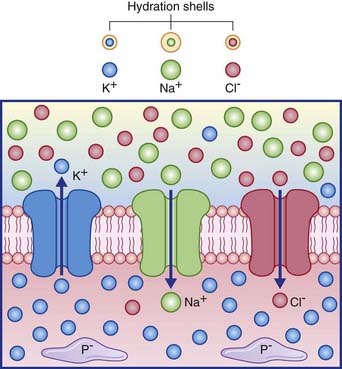
Figure 7.2 depicts the three passive channels concerned with generating the resting potential.
The resting membrane potential
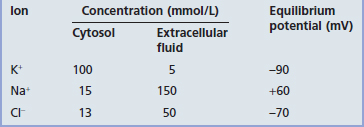
The membrane potential of the resting (inactive) neuron is generated primarily by differences in concentration of the sodium (Na+) and potassium (K+) ions dissolved in the aqueous environments of extracellular fluid (ECF) and cytosol. In Table 7.1, it can be seen that potassium is 20 times more concentrated in the cytosol; sodium is 10 and chloride 3.8 times more concentrated in the ECF.
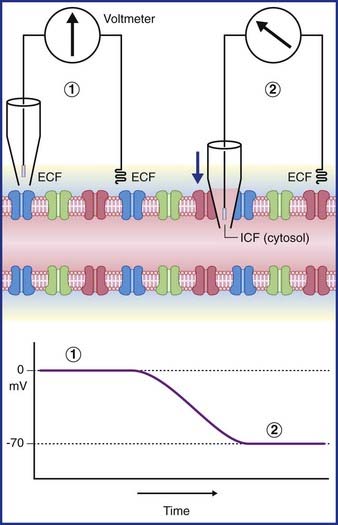
In Figure 7.3, a voltmeter is connected to electrodes inserted into the ECF surrounding an axon. One of the electrodes has been inserted into a glass pipette having a minute tip. On the left side of the figure, both electrode tips are in the ECF, and there is no voltage difference; a zero value is recorded. On the right side, the pipette has been lowered, puncturing the plasma membrane of the axon and admitting the intracellular fluid of the cytosol. The electrical charge now reveals a potential (voltage) difference of −70 mV. In practice, the membrane potential ranges from −60 mV to −80 mV in different neurons. These values represent the resting membrane potential, i.e. when impulses are not being conducted.