Figure 91.1. A schematic for a double- blind, placebo-controlled trial that has been adopted for two recent multicenter brain stimulation trials (Medtronic stimulation of the anterior nucleus of the thalamus [SANTE] trial and NeuroPace responsive neurostimulator system [RNS] trial). Specifically, well-designed clinical trials have control and active therapy arms with patients randomized to therapy ON or OFF. The patient and treating physicians are blinded to this information in order to limit bias.
To determine the efficacy of a particular brain stimulation paradigm (target of stimulation, timing of stimulation, stimulation parameters, etc.) for the treatment of epilepsy requires an appropriate study design. Pilot studies in a small number of patients are often used to initially investigate the safety, feasibility, and evidence of possible efficacy. Pilot and feasibility studies are not adequately powered to prove efficacy but should also use an appropriate design with controls and blinding. Figure 91.1 is a schematic for a double-blind, placebo-controlled trial that has been adopted for two recent multicenter brain stimulation trials (Medtronic stimulation of the anterior nucleus of the thalamus [SANTE] trial and NeuroPace responsive neurostimulator system [RNS] trial; see Figs. 91.2–91.4). As discussed in the following sections, a number of studies that reported positive results have not held up in better designed, more rigorous studies with a placebo-controlled arm.
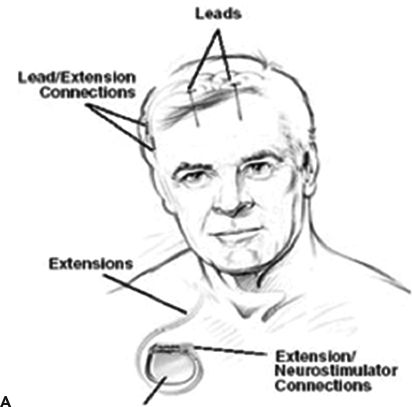
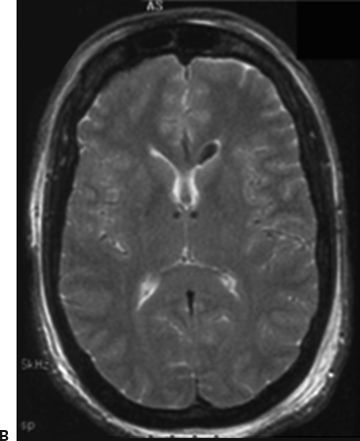
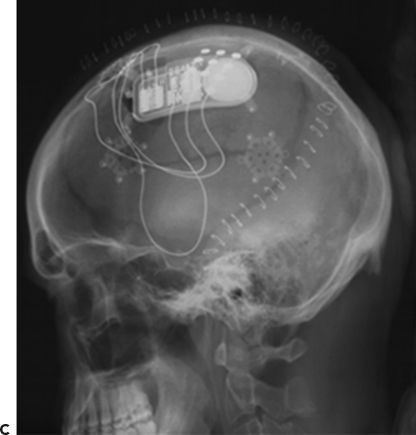
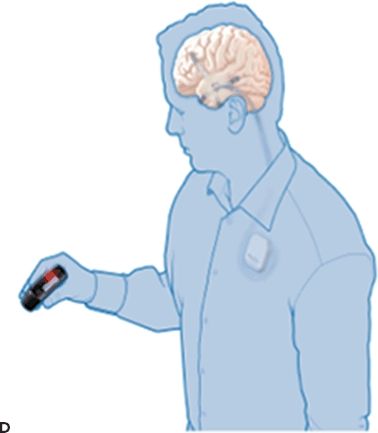
Figure 91.2. Epilepsy device trials. A: Stimulation of the anterior nucleus of the thalamus (SANTE). Schematic showing implanted anterior nucleus of thalamus (ANTS) electrodes and subclavicular generator; B: MRI showing bilateral ANTS electrodes (courtesy of Gordon Baltuch); C: Responsive neurostimulator system (RNS) consists of a neurostimulator implanted into the cranium and subdural electrodes; D: Seizure advisory system (SAS) schematic showing implanted subdural electrodes, subclavicular EEG telemetry unit, and external personal advisory device.
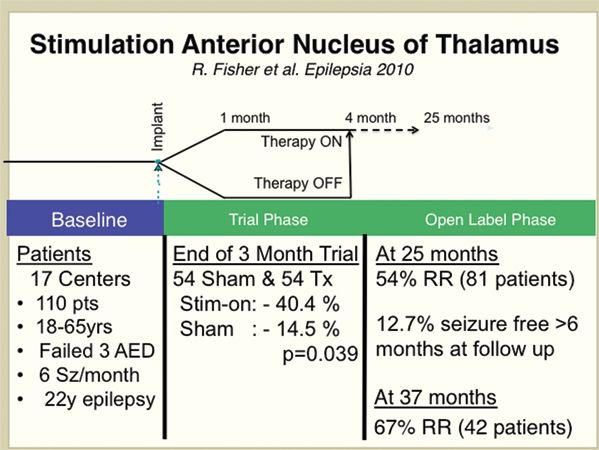
Figure 91.3. The results from a large multicenter, randomized, controlled trial of stimulation of the anterior nuclei of thalamus for epilepsy (SANTE) using the Medtronic DBS device (15).
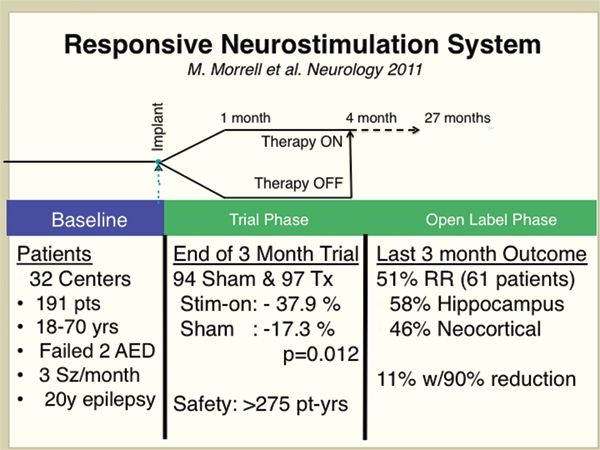
Figure 91.4. The results from a large multicenter, randomized, controlled trial for hippocampal and neocortical responsive stimulation using the NeuroPace RNS device (16).
Baseline
The baseline seizure frequency is determined from the patient diary in a defined period prior to the intervention under investigation. Studies of epilepsy, whether they are drug studies or brain stimulation, typically rely on the patient diary for determining seizure frequency. The reliability of patient reporting is a recognized weakness. Although there are currently no tools for reliably counting seizures in the outpatient setting, a recent study presented data from an implanted seizure advisory system (SAS), which could potentially be used for seizure counting (14).
Implantation
The device is implanted in patients who have met the enrollment criteria of the study. For example, only patients who had the required number of seizures in the 3-month baseline seizure frequency phase are implanted. In order to minimize the acute effect of implantation, there is typically a period of time (approximately 1 month) prior to randomization to stimulation ON/OFF and commencing the blinded treatment phase.
Randomization and Placebo Control
In order to rigorously differentiate the effect of electrode implantation, placebo, and stimulation, a sham surgery arm would be required. In most cases, it is not ethically possible to include sham implantation surgery. The placebo response and efficacy of stimulation are determined by randomization of patients to therapy ON or OFF. In effect, a coin toss (heads/tails) determines whether stimulation is activated or remains inactivated after surgery. In this way, approximately half the patients in the trial are randomized to therapy ON or OFF.
Double-Blind Design
The placebo response is well established in clinical trials and can have a powerful impact on the patient and physician’s perception of treatment efficacy. By blinding the patient and physician to the treatment information, that is, stimulation ON or OFF, the placebo response can be determined. The efficacy of stimulation can be evaluated by directly comparing seizure frequency with stimulation ON versus OFF. A statistically significant reduction in seizures in the stimulation arm versus placebo (i.e., control) can be attributed to the stimulation therapy. Any seizure reduction occurring in the control arm is attributed to placebo response, chance, or possibly implantation effect. As mentioned in the two completed device trials (Medtronic SANTE and NeuroPace RNS), the implantation of the electrodes could conceivably create a therapeutic lesion. Yet, the acute therapeutic effect of electrode implantation appears to wear off with time.
Crossover
A crossover design allows patients who were initially randomized to therapy OFF to receive stimulation after completion of the double-blind study phase. The multicenter trials discussed in the following sections have utilized this single crossover design. In a double crossover study design, patients receiving therapy (ON) are crossed over to no therapy (OFF). In this study design, the possible carryover effects of brain stimulation could confound the interpretation of the results. The “wash-out” period for anticonvulsant medications can be easily obtained, but the time required for “wash-out” of the effect of months of brain stimulation is not known.
Open-Label Extension
In the open-label portion of the trial, all patients receive stimulation without blinding. Often in the open-label phase, medications are adjusted or added, so interpretation of results requires caution. In addition, the patients and physician are no longer blinded to the therapy. Interestingly, the two large-scale multicenter clinical trials (see SANTE and RNS studies discussed below) have all shown evidence for increasing efficacy of brain stimulation with duration of time of receiving stimulation. These results must be interpreted with caution since they come from the open-label portion of the trial, but raise the possibility that brain stimulation has a cumulative therapeutic benefit.
Measures of Efficacy
Commonly reported outcome measures are (i) responder rate (RR), defined as the percentage of patients with a 50% or greater reduction in seizures, (ii) mean reduction in seizures from all patients, and (iii) number of seizure-free patients (defined over a specific duration of the trial, e.g., the most recent 3 months of the open-label phase of the trial). In addition, quality-of-life measures are often assessed, for example, Quality of Life in Epilepsy (QOLIE)-89 scale (17).
Measures of Safety
Side effects are categorized as serious or minor and anticipated or unanticipated. For example, an intracranial hemorrhage associated with electrode placement would be a serious, but anticipated, complication.
Electrical Stimulation Paradigms
The parameter space defining the range of stimulation variables is large and includes the type of stimulation (constant current vs. constant voltage), amplitude, stimulation waveform, frequency, duration, etc. The paradigms of stimulation can be broadly categorized as follows.
Open-Loop Stimulation (Duty Cycle Stimulation)
To date, the majority of stimulation systems utilize duty cycle stimulation. The stimulation is given regardless of the occurrence of seizures or brain activity. For example, deep brain stimulation (DBS) for tremor and open-loop stimulation protocols for the SANTE trial.
Closed-Loop Stimulation (Automated or Responsive Stimulation)
Recently developed systems utilizing implantable microprocessors make it possible for programmable stimulation to be delivered in response to recorded electrophysiologic signals. The NeuroPace RNS system is a closed-loop device capable of recording continuous iEEG and delivering therapeutic stimulation based on automated detection of pathologic activity and seizures.
Control Law Stimulation (Feedback Control Stimulation)
Based on the hypothesis that seizures occur out of a particular brain state that can be characterized by some observable (e.g., features of iEEG), it may be possible to actually prevent seizures by continuously adjusting a therapy (such as stimulation) that is determined by the measured observable. This approach is commonly used in a wide range of engineering applications and has been applied to animal models of epilepsy (18–20).
STIMULATION TARGETS IN THE HUMAN NERVOUS SYSTEM
Intracranial Stimulation
The idea of using electrical stimulation to treat epilepsy has a long history (1,2,21). The following is a discussion of earlier studies with a focus given to studies with good clinical design. The ability to accurately and safely implant electrodes into human brain has led to dramatically successful therapies for some neurologic disorders such as tremor (1). Less successful has been the application of DBS, hippocampus stimulation, and neocortical stimulation for the treatment of epilepsy. Nonetheless, the field has advanced. Two well-designed multicenter clinical trials (Medtronic SANTE and NeuroPace RNS) investigating the feasibility, safety, and efficacy of brain stimulation for treatment of medically resistant focal epilepsy have recently been reported and will be discussed in the following sections.
Cerebellar Stimulation
The cerebellum provides inhibitory outflow and for this reason was an early candidate target for electrical stimulation to treat epilepsy (7,22). In early uncontrolled studies, cerebellar stimulation was reported to yield significant reductions in seizures. An early uncontrolled trial of 115 patients reported 31 patients became seizure free and 56 improved significantly (23). This was a remarkable result and generated considerable interest. However, in a later controlled, double-blinded study of 12 patients, only 2 patients showed improvement (10). The small number of patients studied in the trial limits the ability to draw conclusions.
Caudate Nucleus Stimulation
Chkhenkeli and Chkhenkeli (24) reported a decrease in “interparoxysmal activity” in neocortical and mesial temporal epileptic foci in patients with stimulation of caudate nucleus, but clinical seizures were not investigated.
Mammillary Nuclei
Mirski and Fisher (25) reported an increase in the seizure threshold for pentylenetetrazol-treated rats. However, trials have not been performed in humans.
Centromedian Nucleus of the Thalamus
The CMN is implicated as part of the circuit involved in the generation of spike-and-wave discharges in generalized epilepsy (26). Early studies from Velasco et al. reported significant reductions in seizures for patients with generalized convulsive seizures and atypical absence seizures, but no benefit in patients with complex partial seizures (27,28). Fisher et al. (11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








