Figure 8.1. Electrode positions and nomenclature of the combinatorial 10-10 system proposed by the American Electroencephalographic Society (1) and the International Federation of Clinical Neurophysiology (2).
For consistency and ease of interpretation, we displayed most tracings with the same longitudinal bipolar montage (Fig. 8.2). Occasionally, the activity was best shown with a transverse bipolar montage (Fig. 8.3), a longitudinal bipolar montage with anterior temporal or sphenoidal electrodes (see Fig. 8.2), or a referential montage (Fig. 8.4).
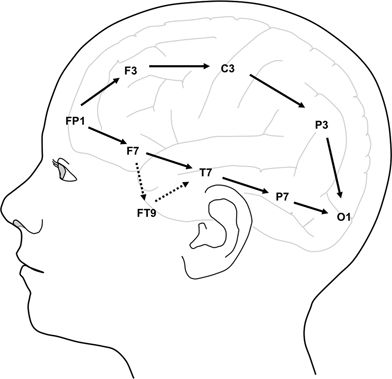
Figure 8.2. Longitudinal bipolar montage, left-sided electrodes. The “double-banana” montage used for almost all the tracings in this atlas includes the channels shown with filled arrows, ordered as follows: right temporal chain, left temporal chain, right parasagittal chain, and left parasagittal chain. The “anterior temporal” montage used in some of the tracings is modified to include the channels shown with broken arrows to reflect anterior, basal, or mesial temporal discharges with anterior temporal (FT9, FT10) or sphenoidal (SP1, SP2) electrodes.
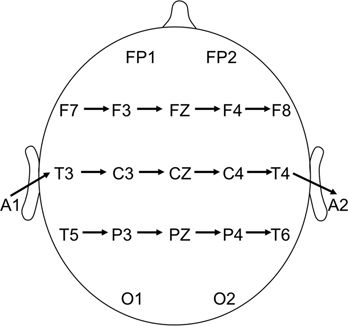
Figure 8.3. Transverse bipolar montage, vertex view. Channels are arrayed in order, as follows: frontal chain, temporocentral chain, and parietal chain.
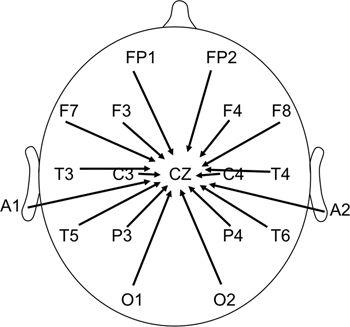
Figure 8.4. Reference montage to vertex.
PART I: NORMAL ELECTROENCEPHALOGRAPHIC PATTERNS AND VARIANTS SOMETIMES CONFUSED WITH EPILEPTIFORM ACTIVITY
For epileptologists to fulfill the basic obligation to “do no harm,” they must avoid “overreading” normal variants on EEG (5,6). This section includes several normal patterns that may be easily mistaken for epileptiform discharges, resulting in an incorrect diagnosis of epilepsy and inappropriate recommendations for antiepileptic medication.
| Small sharp spike | Figure 8.5 |
| 14- and 6-Hz positive spikes | Figure 8.6 |
| 6-Hz “phantom” spike and wave | Figure 8.7 |
| Wicket spikes | Figure 8.8 |
| Subclinical rhythmical electrographic discharges of adults | Figure 8.9 |
| Rhythmic temporal theta bursts of drowsiness | Figure 8.10 |
| Hypnagogic hypersynchrony | Figure 8.11 |
| Vertex wave and positive occipital sharp transients of sleep (POSTS) | Figure 8.12 |
| K-complex and vertex wave | Figure 8.13 |
| Hyperventilation effect | Figure 8.14 |
| Photic driving | Figure 8.15 |
| Breach rhythm | Figure 8.16 |
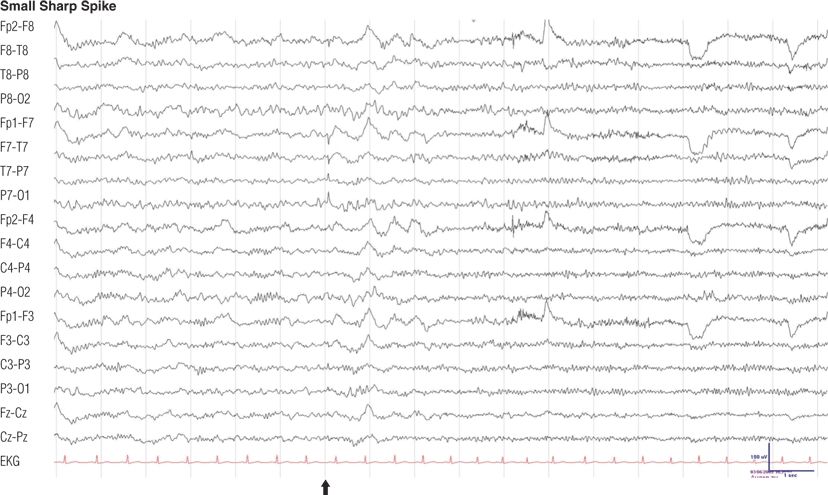
Figure 8.5. Forty-two–year-old man, otherwise normal, with chronic tension headache. Note the low-amplitude monophasic sharp transient (arrow) followed by a minimal slow wave, maximum negativity in the left posterior temporal region (electrode P7), undisturbed background rhythms, during light sleep. During the second half of the EEG, the patient is awake. Small, sharp spikes have also been called benign epileptiform transients of sleep (7).
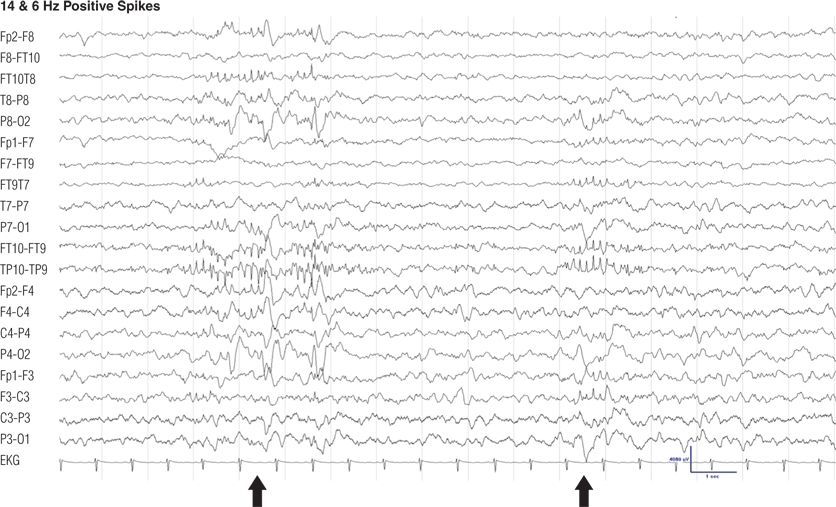
Figure 8.6. Thirteen-year-old girl, otherwise normal, with vasovagal syncope. Note the burst of sharply contoured 14- and 6-Hz activity with maximum positivity in the left and right posterior temporal regions, occurring in light sleep (8). The transhemispheric montage TP10-TP9 depicts the highest amplitude of the positive spikes. Positive spikes of 14 and 6Hz have also been called ctenoids.
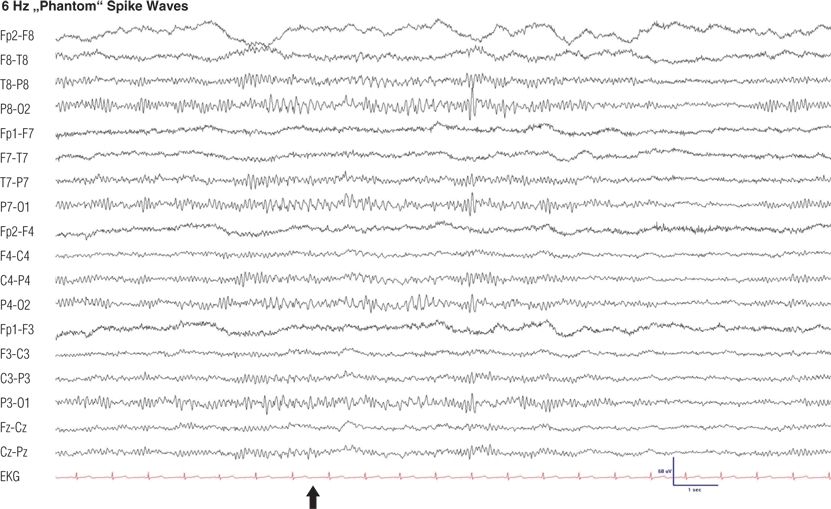
Figure 8.7. Twenty-two–year-old woman with vasovagal syncope. Note the generalized burst of 6-Hz low-amplitude spikes with 6-Hz slow waves occurring during drowsiness in the posterior regions (6).
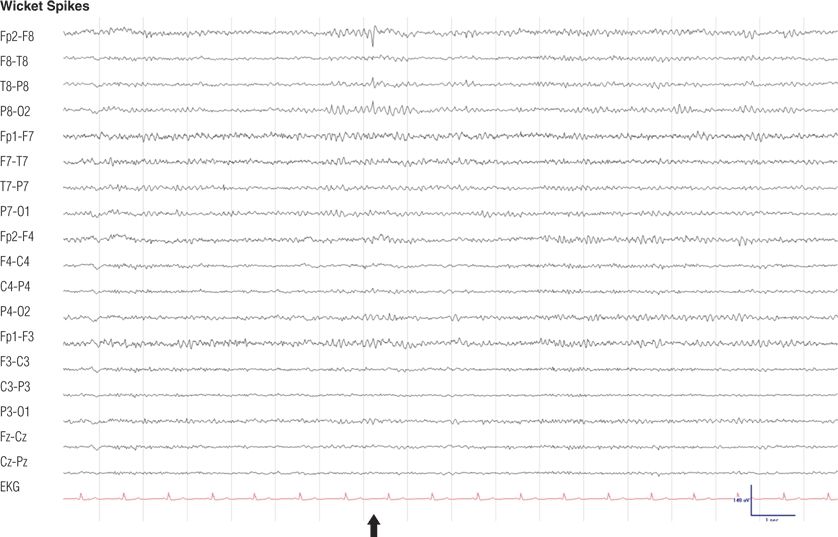
Figure 8.8. Fifty-four–year-old woman with migraine. Note the 7-Hz, rhythmic, sharply contoured waves with maximum negativity in midtemporal regions, occurrence during drowsiness, and undisturbed background rhythms. The typical frequency of wicket spikes is 6 to 11 Hz (9).
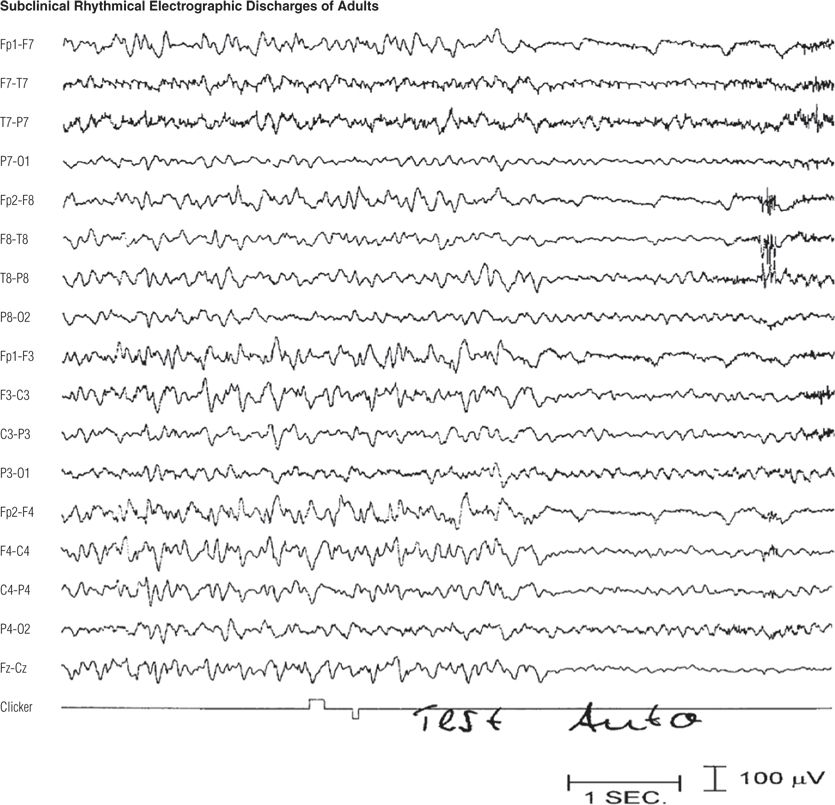
Figure 8.9. Sixty-one–year-old woman with depression. Note the diffuse frontal-maximum, rhythmic, sharply contoured theta and delta activity (10) that ended after 34 seconds and was immediately followed by a normal posterior dominant alpha rhythm. During the rhythmic activity, the patient responded appropriately to an auditory stimulus (clicker). In the last channel, the upward deflection was from the technician’s sound stimulus, and the subsequent downward deflection was from the button pressed by the patient in response. The patient remained awake and responsive throughout the recording and afterward recalled the test word (“auto”).
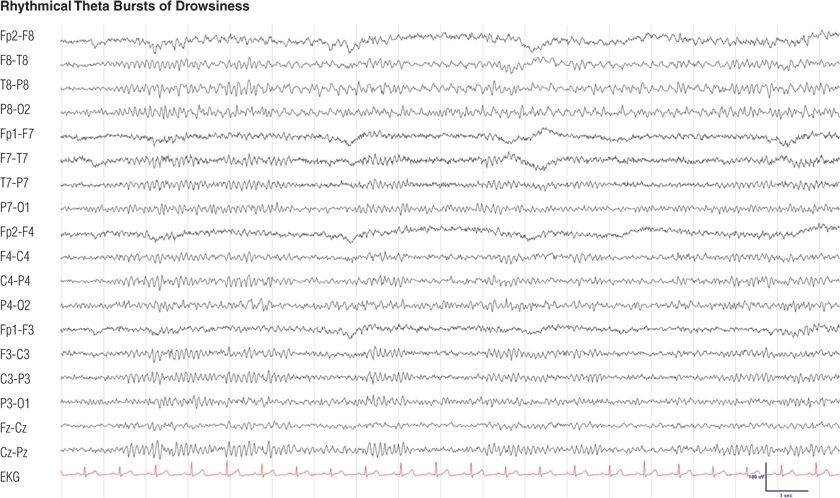
Figure 8.10. Thirty-three–year-old woman with depression. This rhythmic theta activity during drowsiness, with sharply contoured waves maximal in the left midtemporal region, has also been called psychomotor variant (6,11).
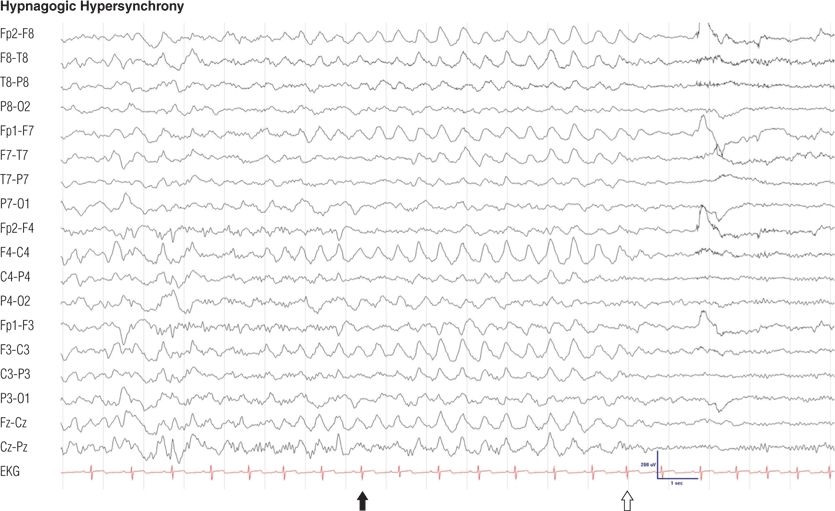
Figure 8.11. Eleven-year-old normal boy. Note the generalized, rhythmic, high-amplitude delta waves during drowsiness (black arrow), which cease with arousal (open arrow).
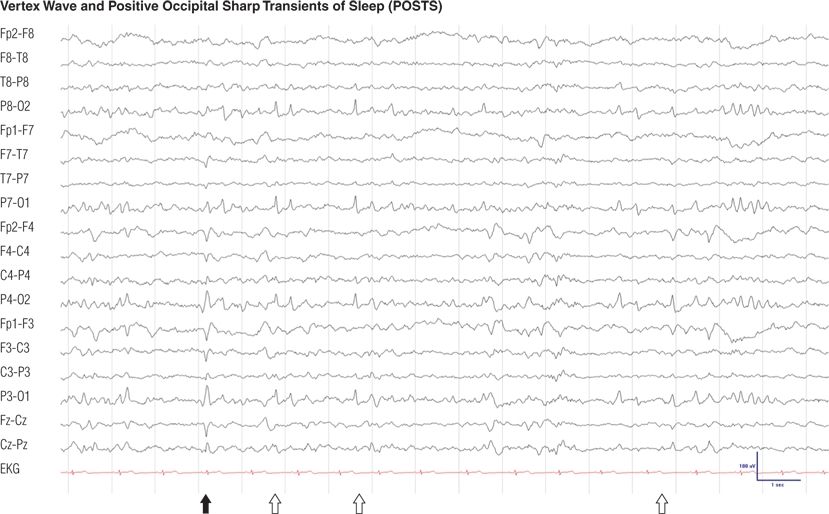
Figure 8.12. Sixteen-year-old girl with vasovagal syncope. Note the central vertex waves (black arrow) and runs of positive occipital sharp transients of sleep (POSTS) (open arrows) (channels 4, 8, 12, and 16), both normal features of stage I or II non–rapid eye movement (non-REM) sleep.
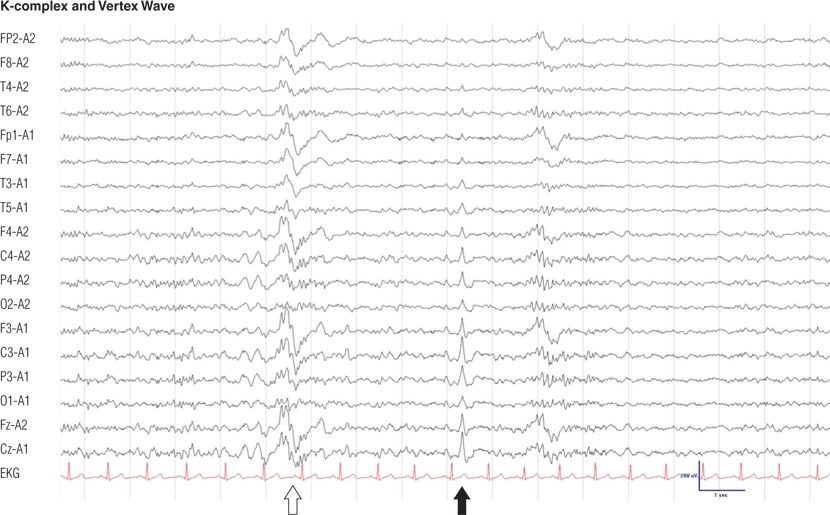
Figure 8.13. Twenty-four–year-old man. K-complexes (open arrow) and vertex wave (solid arrow). Note the sleep spindle, which is associated with the K-complex during stage II non-REM sleep.
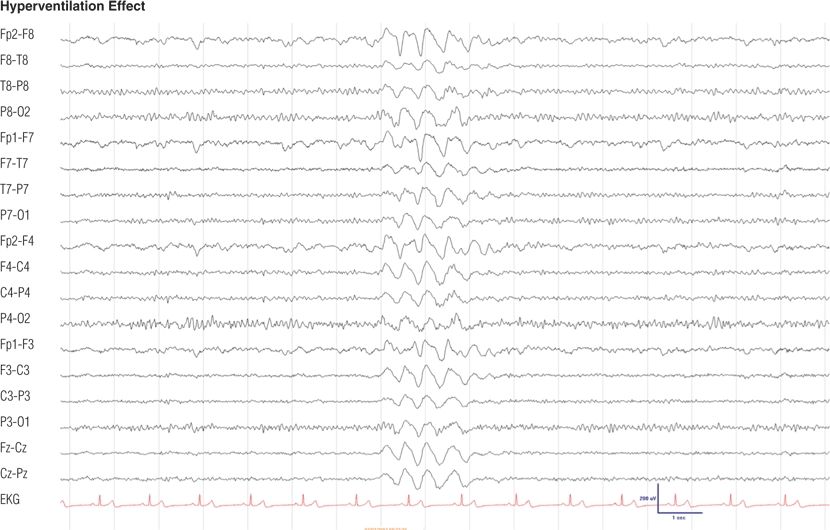
Figure 8.14. Eighteen-year-old girl with school tension headache. Hyperventilation-induced high-amplitude rhythmic delta slowing is a normal response. The girl was alert and responsive during this tracing, which was obtained after 1 minute of hyperventilation.
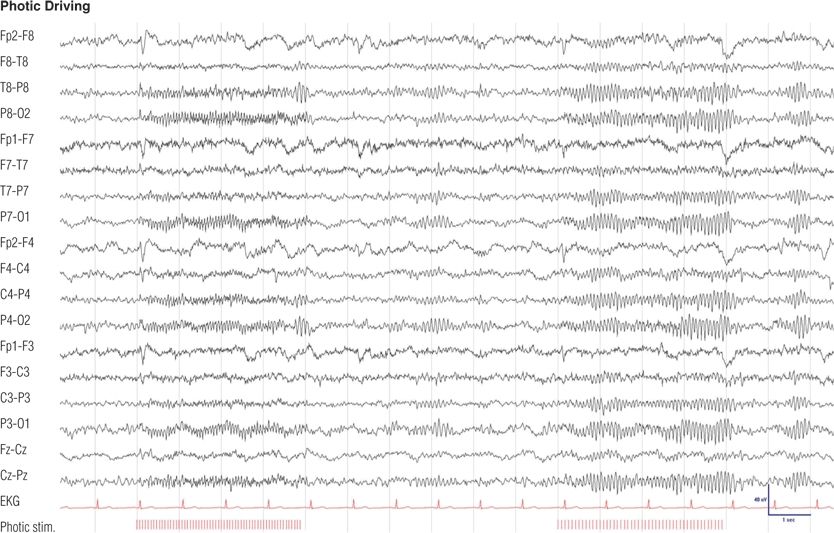
Figure 8.15. Sixteen-year-old boy with childhood absence epilepsy since 7 years of age, seizure free on medication for the last 3 years. Note the time-locked, unsustained, bioccipital response to 8- and 4-Hz photic stimulation, separated by normal posterior background activity. Photic driving represents a normal response to photic stimulation and is not related to the epilepsy of the patient.
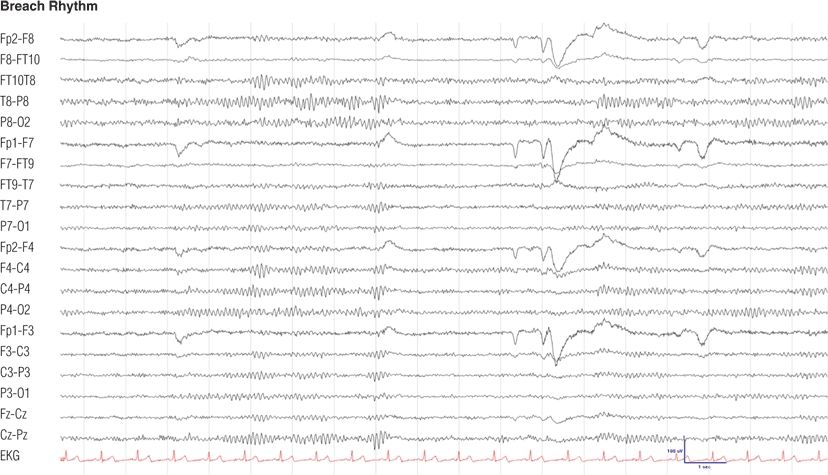
Figure 8.16. Fifty-one–year-old woman with trigeminal neuralgia, status after right parietotemporal craniotomy for vascular decompression. Note the asymmetry of background rhythms owing to the skull defect (12), maximum at the right temporal (T8, P8) electrodes.
PART II: ELECTROENCEPHALOGRAPHIC ABNORMALITIES OF THE GENERALIZED EPILEPSIES
Childhood Absence Epilepsy
| Generalized spike–wave complexes | Figure 8.17 |
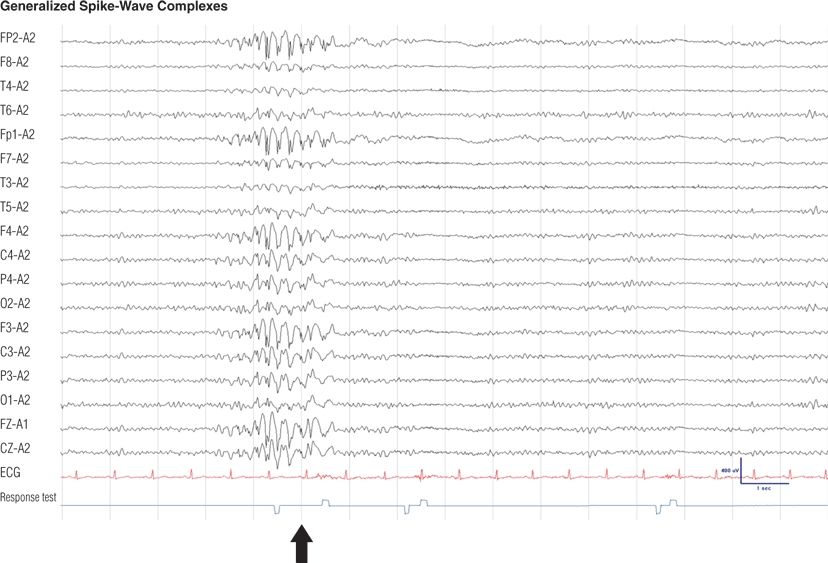
Figure 8.17. Twelve-year-old boy, otherwise normal, with recent onset of absence seizures. These irregular generalized spike-and-wave complexes were precipitated by hyperventilation and lasted for almost 2 seconds. Note that the patient failed to press a button (black arrow) as a response to an auditory stimulus (clicker test) given to him during the SWC, but he responded to a second stimulus at the end of the discharge. These episodes would go unnoticed if not tested.
Juvenile Absence Epilepsy
| Absence status epilepticus | Figures 8.18 and 8.19 |
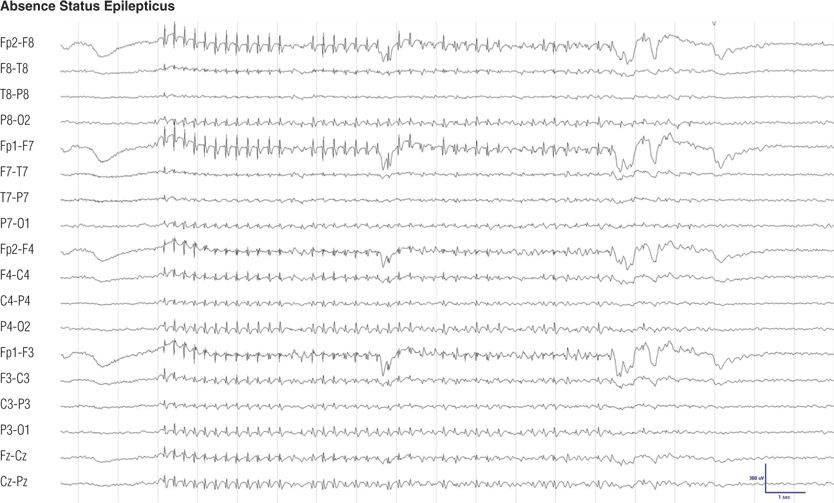
Figure 8.18. Thirty-six–year-old woman with absence seizures during childhood. She was seizure free throughout adulthood off medication until recently absence status epilepticus began during drug treatment with imipramine for depression. During this episode, with generalized spike-and-wave complexes, she was unresponsive. Electroencephalographic findings and behavior returned to normal after intravenous injection of lorazepam.
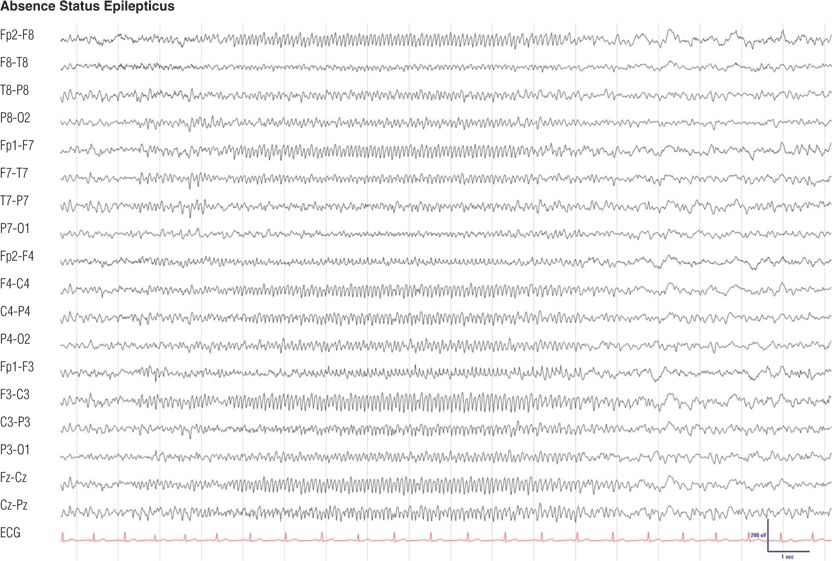
Figure 8.19. Fifty-eight–year-old man with juvenile absence epilepsy with a 2-day history of confusion and agitation prior to this electroencephalogram (EEG). He was switched to topiramate and stopped valproic acid erroneously. EEG findings and behavior returned to normal after intravenous injection of clonazepam.
Myoclonic Epilepsy
| Photoparoxysmal response in juvenile myoclonic epilepsy | Figure 8.20 |
| Generalized myoclonic seizure | Figure 8.21 |
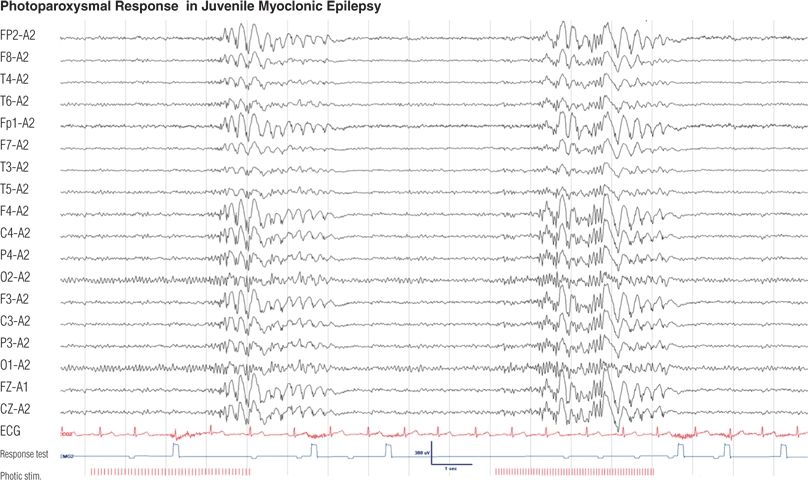
Figure 8.20. Sixteen-year-old boy with a 4-year history of myoclonic jerks of the upper extremities and a few generalized tonic–clonic seizures in the morning after awakening. Photic stimulation elicited generalized polyspikes and spike–wave complexes at 12 and 15 Hz. His responses to auditory stimuli during these discharges were either absent or delayed (absence seizure) (see clicker test).
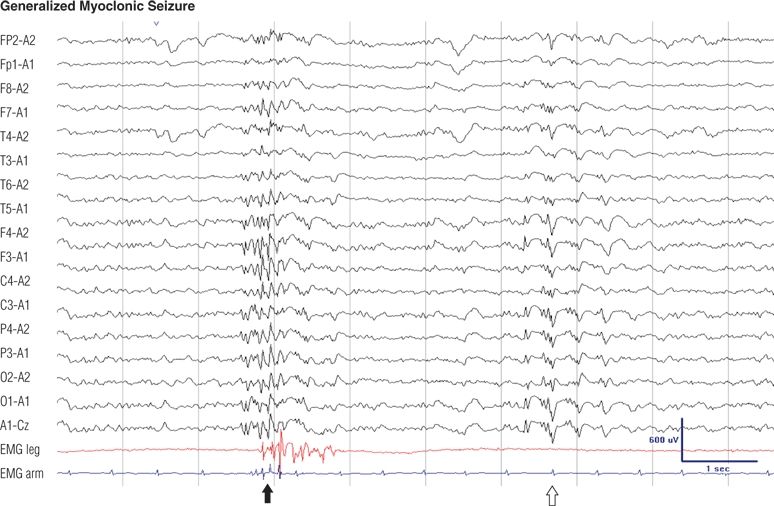
Figure 8.21. Twenty-two–year-old man, otherwise normal, with generalized myoclonic and generalized tonic–clonic seizures on awakening since adolescence. His generalized myoclonic seizures consisted of jerks of the arms. EEG during drowsiness. Note the dense generalized polyspikes were associated with jerks (solid arrow), whereas the other discharges (open arrow) were not.
Infantile Spasms
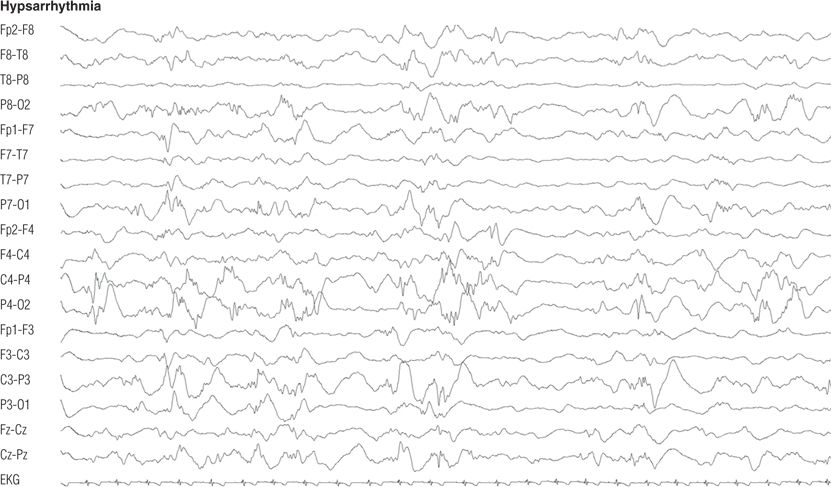
Figure 8.22. Ten-month-old boy with infantile spasms and developmental delay. Awake electroencephalographic record showed disorganized background rhythms dominated by multifocal spikes and high-amplitude slowing.
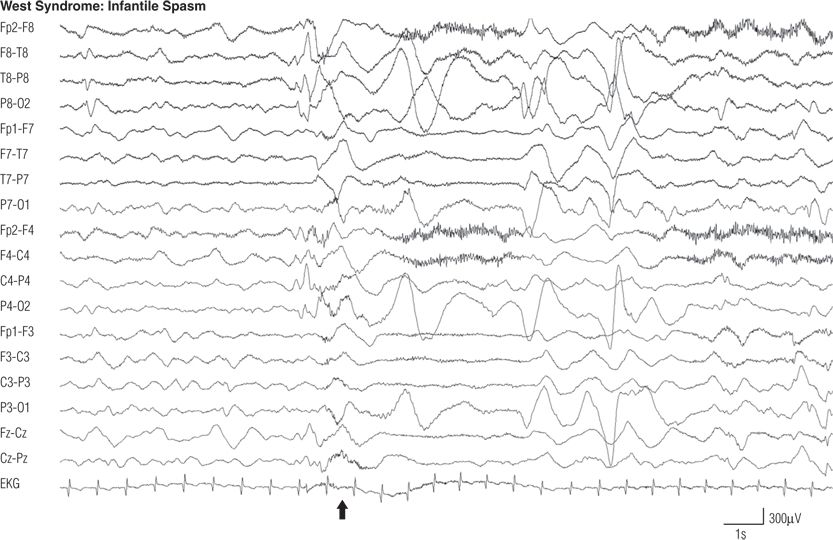
Figure 8.23. EEG during a spasm (arrow), from the same infant as in Figure 8.22. Note the generalized electrodecremental pattern for 3 seconds. The spasm involved tonic abduction and extension of both arms with flexion of the trunk and neck.
Lennox–Gastaut Syndrome
| Generalized slow spike–wave complexes | Figure 8.24 |
| Generalized paroxysmal fast and polyspikes in sleep | Figures 8.25 and 8.26 |
| Atonic seizures | Figure 8.27 |
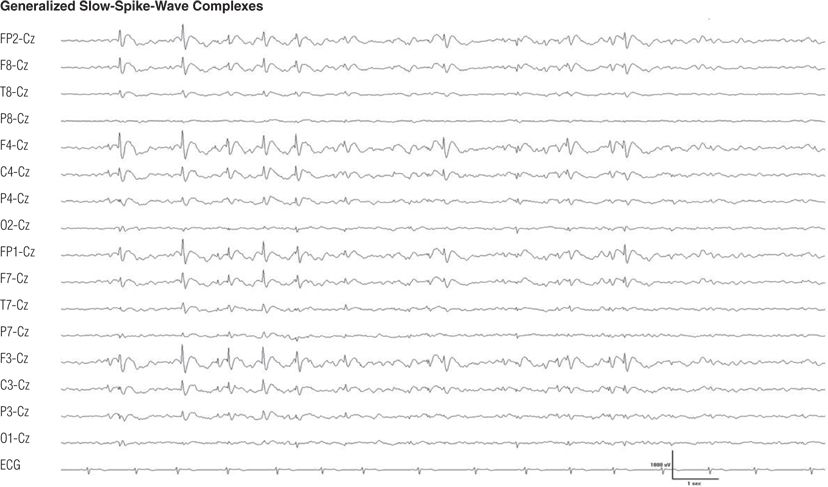
Figure 8.24. Seven-year-old boy with developmental delay and intractable generalized tonic, atonic, myoclonic, tonic–clonic, and atypical absence seizures since age 3 years. Note the bifrontal maximum of the generalized sharp– and slow–wave complexes (5), also called slow spike-and-wave complexes.
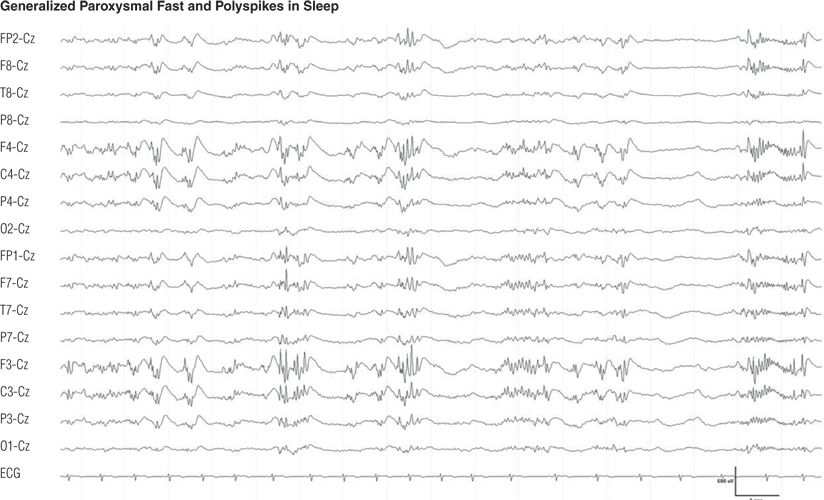
Figure 8.25. Same patient as in Figure 8.24. Generalized polyspikes and paroxysmal fast during drowsiness.
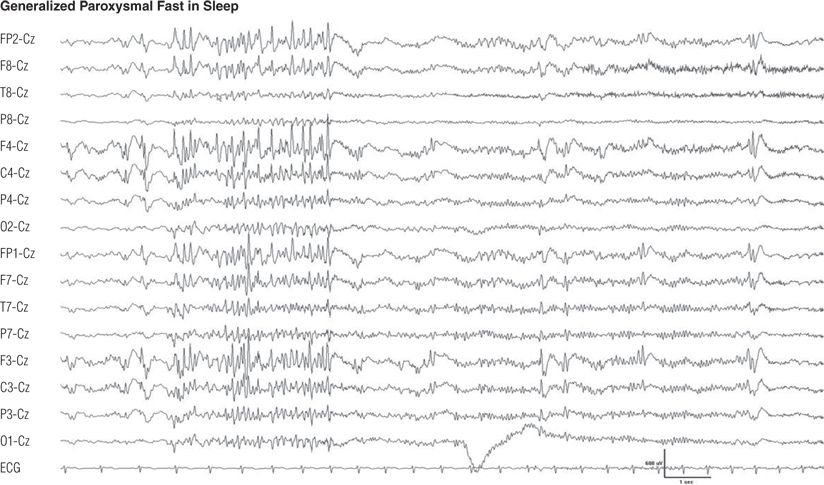
Figure 8.26. Same patient as in Figures 8.24 and 8.25. During drowsiness, generalized paroxysmal fast occurred. When the paroxysmal fast lasted longer than 5 to 6 seconds, generalized tonic seizure was observed.
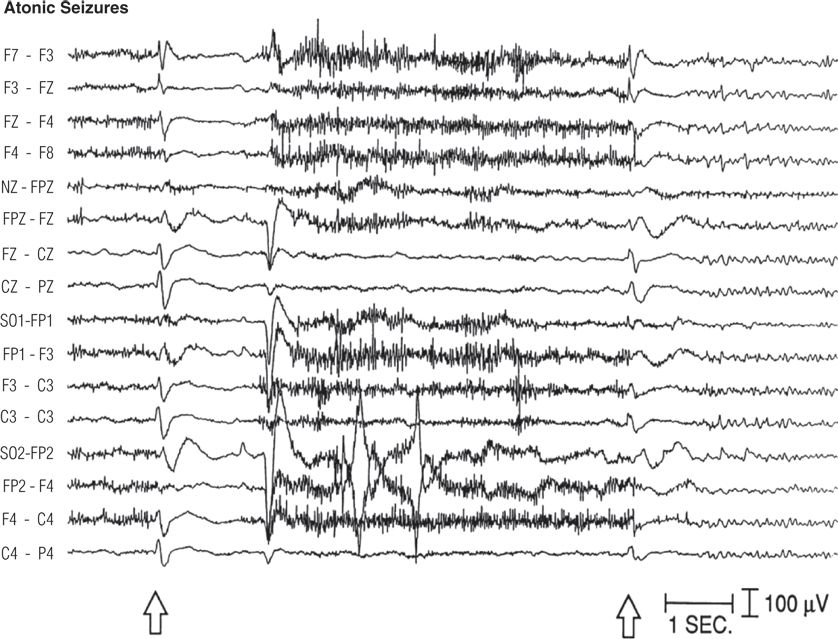
Figure 8.27. Forty-one–year-old man with borderline intelligence and intractable generalized tonic, atonic, generalized tonic–clonic, and atypical absence seizures since age 3 years. Interictal electroencephalogram showed generalized sharp– and slow–wave complexes. Two seizures are recorded here, with limp head nodding plus tonic stiffening and elevation of both arms. Each seizure began with a generalized sharp wave (open arrows) followed by attenuation of electroencephalograph activity and cessation of muscle artifact.
Intractable Epilepsy with Multifocal Spikes
| Intractable epilepsy with multifocal spikes | Figure 8.28 |
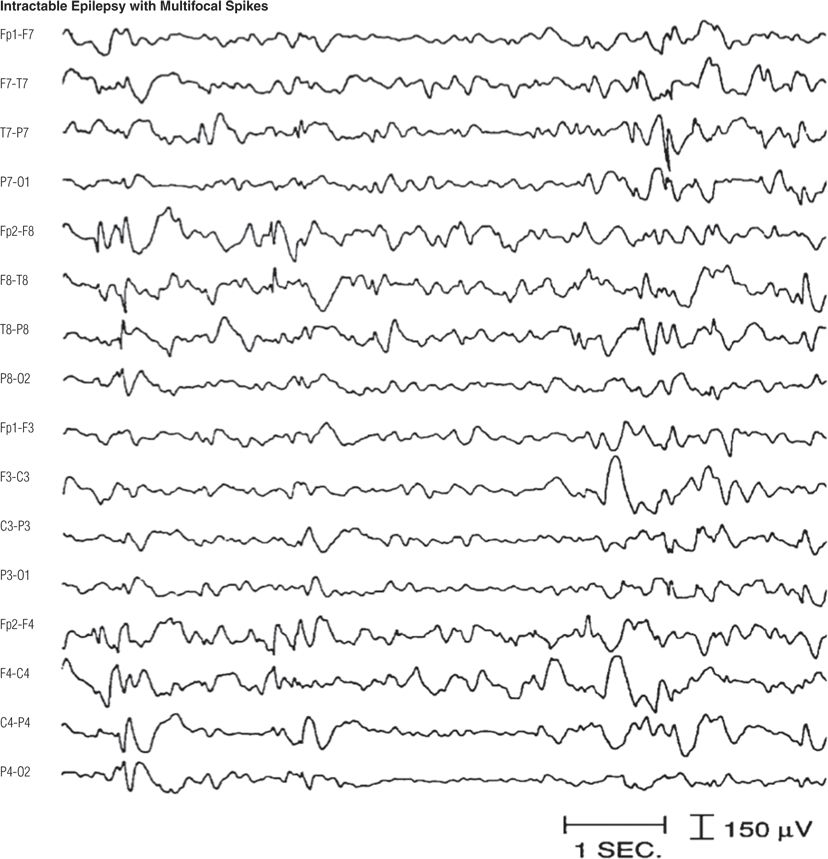
Figure 8.28. Three-year-old boy with developmental delay and intractable clusters of generalized tonic, myoclonic, and atypical absence seizures. This electroencephalographic pattern is not uncommon in children with clinical features similar to those of Lennox–Gastaut syndrome (13).
Stimulation-Related Epilepsy
| Cognitive (Soduko)-induced spikes | Figures 8.29 and 8.30 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








