Chapter 125 Endocrine Disorders
Abstract
There are many diverse associations between human endocrine function and sleep. Importantly, neuroendocrine and metabolic physiology is often influenced by behavioral states of sleep and wakefulness (see Chapter 26). Extensive research in this area has flourished since the development of better assays of endocrine function, paralleling the growth of human sleep research stimulated by the availability of polysomnography. Endocrine rhythms have often been labeled either sleep related (when the predominant change in fluctuation is nocturnal) or circadian (when the rhythm appears to be regulated by an internal clock rather than by periodic changes in the external environment). The predominant influences are intrinsic circadian rhythmicity and sleep, which interact to varying degrees to produce the characteristic 24-hour rhythm of each hormone. On the other hand, aberrations of normal endocrine function may influence sleep or alter state-affected parameters such as breathing or the electroencephalogram (EEG). This chapter concentrates on these changes, limiting discussion to particular endocrine disorders in adults and children.
Acromegaly and Other Growth Hormone Disorders
Acromegaly is a condition of growth hormone (GH) excess in adults, characterized by the insidious development of coarsening of facial features, bony proliferation, and soft tissue swelling. It is usually secondary to a GH-producing pituitary adenoma, which may be either a microadenoma or a macroadenoma. Rarely, the GH excess commences before puberty and closure of the epiphyses, and then the condition is termed gigantism. It occurs with equal frequency in both sexes, with a prevalence of 50 to 70 cases per million. The clinical features may result from the local effects of an expanding pituitary mass in addition to the effects of excess GH secretion, which include disordered somatic cell growth and insulin resistance. The mortality of untreated or partially treated acromegaly is about double the expected rate in healthy subjects matched for age. Upper airway obstruction in acromegaly was first described over 100 years ago.1
Epidemiology and Risk Factors
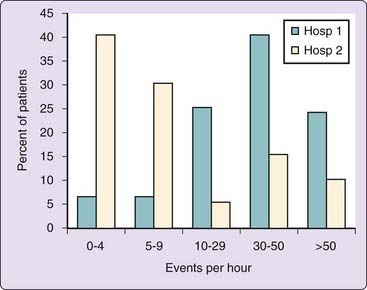
Sleep-disordered breathing is extremely common in patients with acromegaly. Studies have shown that at least 60% of unselected patients with acromegaly have sleep apnea (Fig. 125-1).1 Almost all patients with acromegaly in this series were noted to have heavy snoring. A number of prevalence studies using limited nocturnal respiratory monitoring have also shown a very high prevalence of sleep-breathing disorders in acromegaly.2,3 In keeping with typical sleep apnea epidemiology, abnormal breathing during sleep in patients with acromegaly is associated with increasing age. However, obesity has less influence on the prevalence of sleep apnea in acromegaly.1–3
Pathogenesis
Although it would appear logical that sleep apnea in acromegaly is secondary to macroglossia causing narrowing the hypopharynx, the exact etiology is unclear.1 Endoscopic studies of the upper airway have indicated little posterior movement of the tongue,2 and other studies using cephalometry have produced divergent findings when examining craniofacial anatomy in patients with acromegaly both with and without sleep apnea.4
Patients with acromegaly have a high rate of central apnea, with up to 34% of the total group of patients having sleep apnea in one study.1,5 A waxing-and-waning central apnea pattern of breathing in studies of static charge–sensitive bed has also been reported as more common in patients with acromegaly as opposed to typical upper airway obstruction.2
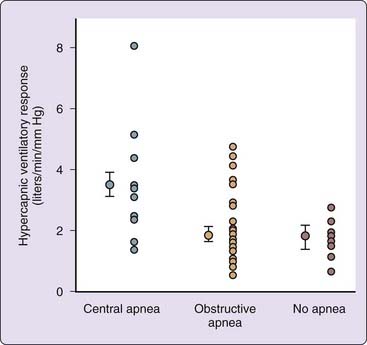
The high prevalence of central apnea in patients with acromegaly suggests that abnormalities of central respiratory control are involved. This has been supported by the finding that patients with central sleep apnea had significantly lower awake arterial CO2 levels and increased ventilatory responsiveness in the central group6 than those with obstructive apnea (Fig. 125-2).1,7 Central apnea occurs in association with a wide range of disorders, and many potential mechanisms have been described, including disordered central respiratory control.7 The precise cause in acromegaly is unclear, but hypotheses include alterations in central somatostatin pathways disinhibiting respiratory control.7 Another mechanism may be an effect of GH on central respiratory control, either directly or by altering metabolic rate, that induces central apnea. This is supported by the correlation between GH hypersecretion and the prevalence of central apnea.1,7 Interestingly, apparent central apneas have been observed in Beagles exposed to medroxyprogesterone, which in turn causes GH increases and an acromegaly-like condition.7
Disease Activity in Acromegaly and Sleep Apnea
Most studies have documented persisting sleep apnea despite treatment of acromegaly by pituitary surgery. Moreover, no clear correlation has been found between disease activity and sleep apnea severity, and no significant differences in mean GH and IGF-1 levels in patients with and without sleep apnea were observed.1 Using more extensive GH measurements in patients with acromegaly, no significant differences in mean GH and GH pulsatility were found between patients with and without sleep apnea.1 In contrast, others8 have reported lower GH levels in patients with milder sleep apnea. Studies using octreotide, a somatostatin analogue, have shown powerful GH reduction with a parallel decrease in apnea severity.5 However, even in this study, there was no relationship between the decrease in apnea and the decrease in GH levels.5
At present, it is not known what proportion of patients with both sleep apnea and acromegaly will have complete resolution of their sleep apnea after cure of acromegaly. This will require careful prospective studies that accurately monitor true cure of acromegaly. However, it is clear that sleep apnea does occur in cured acromegaly.1,5 The combination of inactive acromegaly and sleep apnea may occur for a number of reasons. First, sleep apnea is a common disorder and may be coincident to acromegaly in patients with other risk factors for sleep apnea. Second, it may take a long time after normalization of GH secretion for the effects of acromegaly to resolve, or there may be permanent effects on upper airway function or sleep-breathing regulation. Although there appears to be no relationship between disease activity and the severity of sleep apnea, patients with central sleep apnea have much higher IGF-1 and fasting GH levels than patients with obstructive apnea.1,5
Morbidity and Mortality of Acromegaly and Sleep Apnea
The adverse health risks of both acromegaly and sleep apnea are well established. Both disorders are associated with an increased risk of hypertension. In acromegaly, the blood pressure level is sometimes reduced by successful transsphenoidal surgery. One postulated mechanism for hypertension in acromegaly is sodium and water retention secondary to GH overproduction. Interestingly, more than 50% of patients with both acromegaly and sleep apnea have hypertension,1 but all patients with acromegaly who did not have sleep apnea were normotensive. Patients who were hypertensive had significantly higher respiratory disturbance indices and a greater degree of sleep hypoxemia than those who were normotensive. Mean 24-hour GH and IGF-1 levels and the degree of obesity were not significantly different in those with hypertension compared with those without hypertension. Using multiple regression, both the respiratory disturbance index and age were found to be independent predictors of hypertension.1 Historically there has been an excess of deaths resulting from cardiovascular and respiratory causes, although the exact cause of the respiratory deaths was unclear. As there is no excess of chronic lung disease in patients with acromegaly, it is likely that the observed respiration-related deaths were the result of upper airway obstruction.
Does Acromegaly Cause Nonrespiratory Sleep Disorders?
Somnolence has long been recognized as part of the clinical spectrum of acromegaly. Early descriptions of sleepiness in patients with acromegaly suggested a link with narcolepsy, but subsequent data support the view that the dominant sleep disorder caused by acromegaly is sleep apnea. A direct effect of increased GH in promoting sleep and sleepiness has been suggested, but, alternatively, sleepiness in the absence of sleep apnea result from the effects of radiotherapy.9
Growth Hormone Deficiency
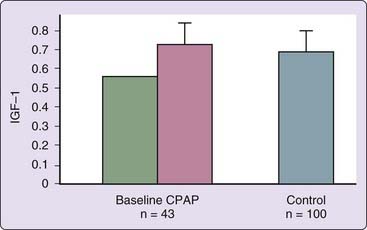
Adults with sleep apnea appear to have relative GH deficiency, which is reversible by nasal continuous positive airway pressure (CPAP) (Fig. 125-3).8,10 This deficiency in untreated patients is probably related to abnormal sleep structure (see Chapter 56) and hypoxemia during sleep. There is no evidence that growth hormone deficiency promotes sleep apnea or other sleep disorders.11
Sex Hormone Disorders
Male Hormonal Disorders
Over the past 2 decades, a number of reports have described the development of sleep apnea in both sexes after testosterone therapy.12 Testosterone was also reported to exacerbate sleep apnea in a 13-year-old boy, associated with an increase in upper airway collapsibility during sleep.6 An important message from these studies is that patients beginning androgen replacement therapy should be questioned closely for sleep apnea symptoms and monitored during the course of their therapy in case such symptoms develop. The possibility of more extensive use of testosterone therapy in eugonadal men for contraception or for “andropause” will probably bring testosterone-induced apnea into clinical practice. Simple, device-based methods of sleep apnea screening may also need to be used in such patients. Larger, definitive placebo-controlled testosterone intervention trials in men with androgen deficiency are required.
Testosterone also influences sleep architecture. In a study of pharmacologically induced hypogonadism,13 with and without gonadal steroid replacement, hypogonadal males had reduced 24-hour prolactin levels and a reduced percentage of stage 4 sleep in the hypogonadal state compared with those receiving testosterone replacement. Melatonin secretion is increased in male patients with gonadotropin-releasing hormone (Gn-RH) deficiency and in low-testosterone hypergonadotropic hypogonadal patients.14 However there are no data on the prevalence of sleep disturbance in these patients. The observation of reduced total sleep time without worsening alertness on task with high-dose testosterone therapy is intriguing in this context.12 Data demonstrating lower testosterone levels in those with sleep disturbances and shift-work sleep disorder15 indicate that the topic of sex steroids and sleep is clearly worthy of more extensive research.
Low testosterone levels have been reported in men with sleep apnea,8 and this appears to be independent of age, degree of obesity, and presence of awake hypoxemia and hypercapnia. In contrast, a cross-sectional population-based study of elderly men found an association between a range of sleep disturbances and low testosterone that were explained largely by body mass index.16 However, testosterone levels increase with treatment of sleep apnea using nasal CPAP or even successful uvulopalatopharyngoplasty.8 These androgen abnormalities in patients with sleep apnea (decreased sex hormone–binding globulin [SHBG] and free and total testosterone) are qualitatively as well as quantitatively distinct from those reported in aging (increased SHBG, decreased free and total testosterone) and obesity (decreased SHBG and total testosterone, normal free testosterone). Importantly, despite the fall in plasma free and total testosterone levels, there was an increase in basal plasma gonadotropin (luteinizing hormone, follicle-stimulating hormone) levels. These findings, together with the retention of pituitary sensitivity to exogenous Gn-RH in sleep apnea,17 point to a hypothalamic abnormality as the cause of the fall in testosterone levels. This is supported by data indicating impaired luteinizing hormone secretion in patients with sleep apnea and partial correction with 9 months of nasal CPAP treatment.18 The potential causes of this hypothalamic abnormality are essentially similar to those involved in reduced GH secretion. Testosterone levels are significantly reduced by sleep deprivation and fragmentation. Therefore, sleep fragmentation in sleep apnea may lead to disruption of sleep-entrained rhythms in luteinizing hormone and testosterone. Sexual dysfunction reported in patients with sleep apnea may be mediated by the sex hormone changes seen in sleep-disordered breathing. The low testosterone levels may also interact with low IGF-1 levels and impair anabolism. Androgens may exacerbate sleep apnea, and it is possible that the fall in androgen levels may be part of an adaptive homeostatic mechanism to reduce sleep-disordered breathing.19 However, androgen-lowering therapy with the nonsteroidal androgen antagonist flutamide did not alter sleep-disordered breathing or awake ventilatory drive.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree