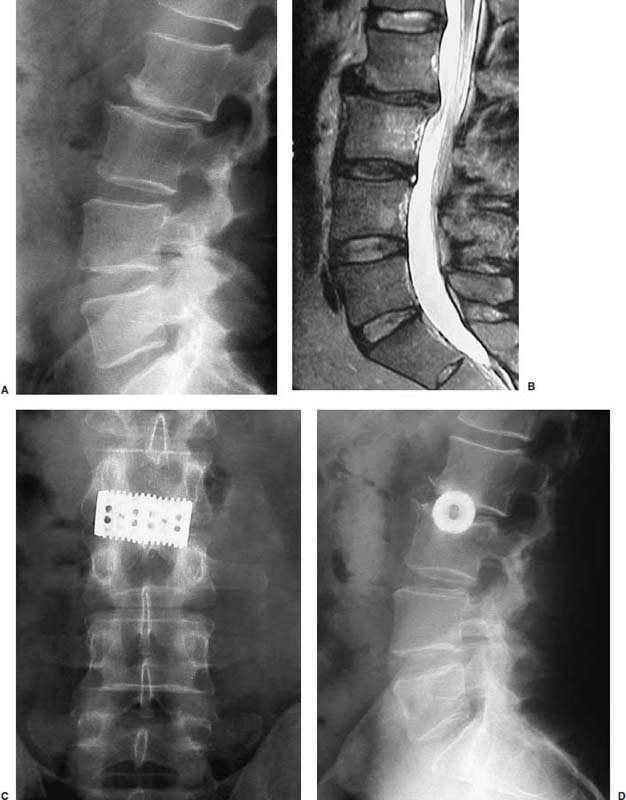
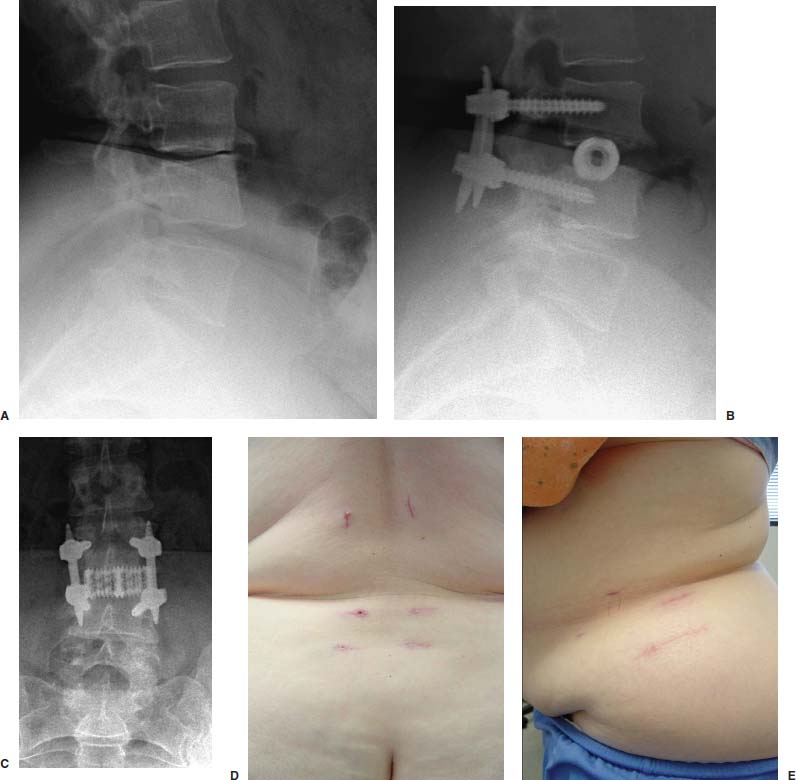
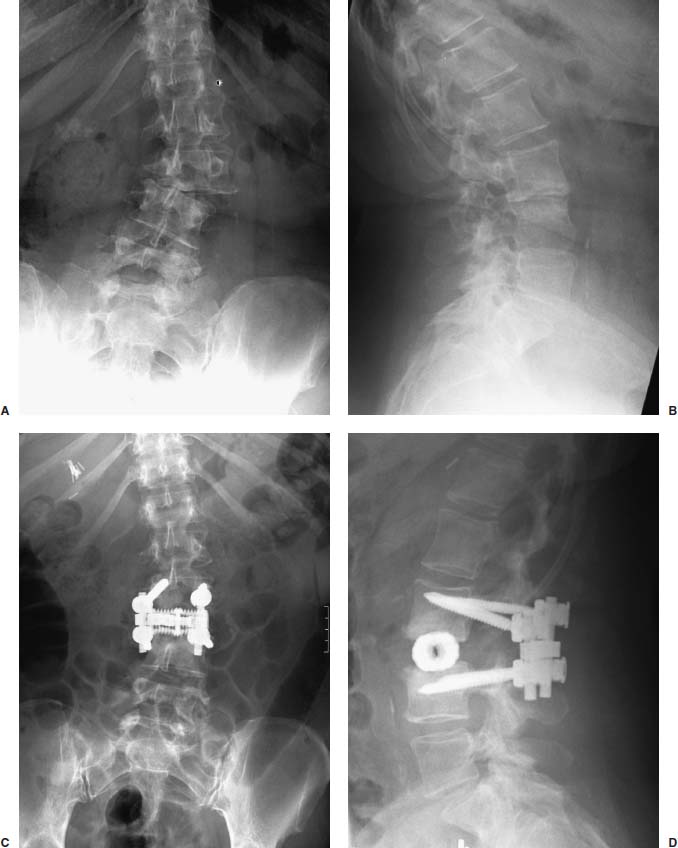
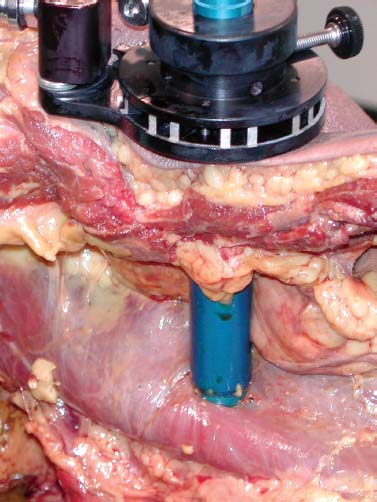
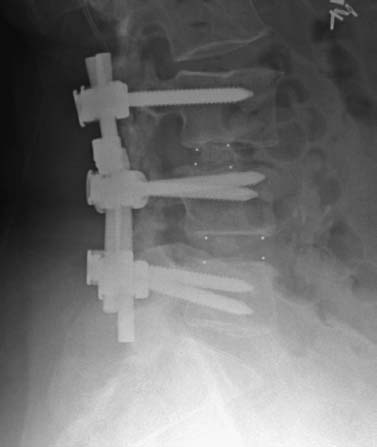
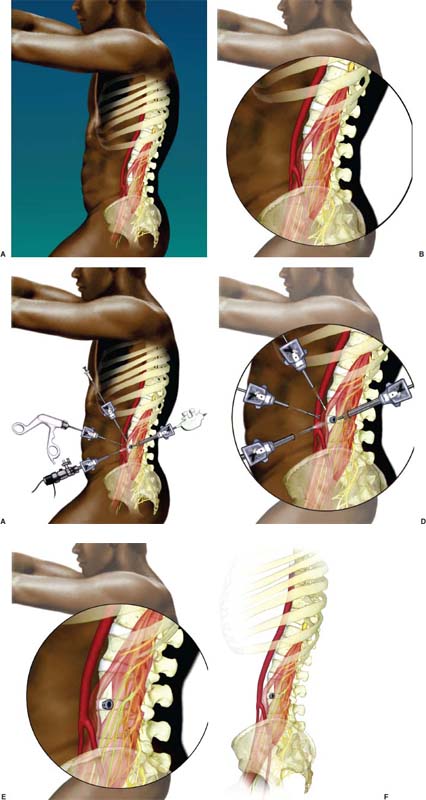
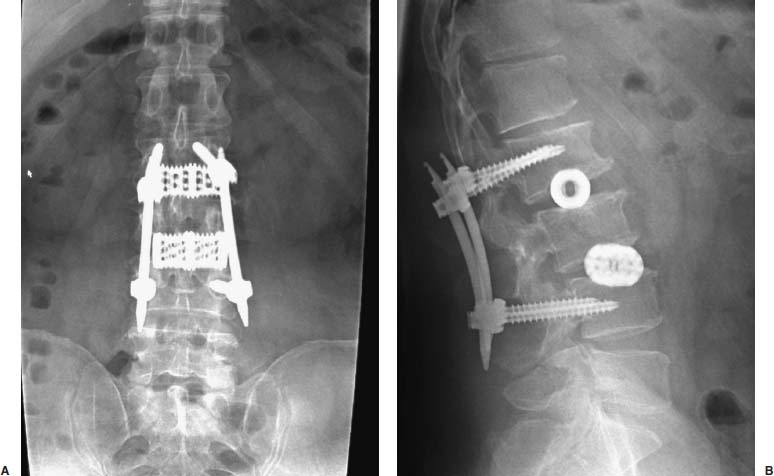
Anterior approaches for lumbar interbody fusion have been increasingly used in an attempt to lower the incidence of pseudoarthroses and to re-create the patient’s normal sagittal alignment.1–9 The majority of complications associated with anterior lumbar interbody fusion (ALIF) are associated with the surgical exposure. Most of these techniques usually require the presence of an experienced general or vascular surgeon due to the risk of serious complications.10,11 Although low, the incidence of injury to the great vessels or sympathetic plexus is not negligible, and the consequences of such potential injuries can be debilitating for the patient.6 Retroperitoneal lumbar fusion and stabilization offers several advantages over conventional anterior approaches. Retroperitoneal approaches eliminate intra-abdominal adhesions that cause bowel obstruction. The lateral retroperitoneal approach eliminates dissection of the great vessels and the sympathetic plexus, minimizing the complications of bleeding or retrograde ejaculation. This approach also minimizes nerve injury because dissection, drilling, and reaming are directed laterally rather than posteriorly toward the spinal canal. It was not until 1992, with the introduction of threaded spinal fusion cages, that laparoscopic spine fusion began to evolve. In 1998, McAfee, Regan, and colleagues5 described a minimally invasive, endoscopic anterior retroperitoneal approach to the lumbar spine with an emphasis on the lateral BAK cage. This technique did not require CO2 insufflation, Trendelenburg positioning of the patient, entrance into the peritoneum, or anterior dissection near the great vessels. Following entry into the retroperitoneal space from a direct lateral approach, the trajectory of this approach is anterior to the psoas muscle, requiring a considerable amount of retraction of the psoas posteriorly. This causes significant muscular swelling and weakness postoperatively. A transpsoas musclesplitting approach through its anterior third provides a more direct approach to the lumbar interbody space and is the approach preferred at this time. Indications The indications for lateral endoscopic transpsoas lumbar fusion are single- or two-level symptomatic degenerative disk disease, segmental spinal instability, progressive lumbar scoliosis, and pseudarthrosis. This approach may be used for lumbar levels 1 through 4. Low-grade spondylolisthesis, two-level degenerative disk disease, and lumbar scoliosis may be corrected with this approach by surgeons with experience (Figs. 27–1, 27–2, 27–3, 27–4, 27–5). Patients with discogenic pain should be selected for surgery based on a history of mechanical symptoms and failed conservative therapy. Patients with a strong mechanical back pain history who have conformed to a physical therapy program for 4 months without relief are good candidates for the procedure. Radiographic findings of disk space narrowing, end-plate sclerosis, and osteophyte formation indicate degenerative disk changes. MRI should provide confirmation of degenerative disk disease with evident Modic changes. Diskography is performed in all patients with more than single-level degenerative disk disease. FIGURE 27–1 This 43-year-old patient presented with intractable mechanical back pain radiating into the anterior thigh. Lateral x-ray (A) and MRI (B) show degenerative changes and retrolisthesis, with no evidence for nerve root impingement. Postoperative x-rays (C,D) show lateral BAK. This patient was treated in a brace for 12 weeks and went on to solid fusion. FIGURE 27–2 This 46-year-old patient with mechanical back pain and demonstrated single-level degenerative disk disease (A) was treated with lateral endoscopic fusion followed by percutaneous Sextant pedicle fixation (B,C). Posterior and lateral skin views (D,E) show healed incisions. Bone graft was taken from the lateral iliac crest, as shown by the larger incision on the lateral view. Contraindications Patients with extensive peritoneal adhesions from previous surgery or inflammatory or infectious disease affecting the peritoneum should be excluded from the lateral endoscopic approach. Patients who have overlying psychological conditions or positive Waddell’s signs or who are habitual narcotics users are not candidates for fusion surgery. Surgical Technique Following the induction of general endotracheal anesthesia, the patient is turned in a right lateral decubitus position (left side up) (Fig. 27–6A,B) on a beanbag on a radiolucent table, with the kidney rest elevated. Anteroposterior and lateral intraoperative fluoroscopy is then used to verify the approximate level of the desired disk space, with a metal marker on the skin in the midaxillary line. This method optimizes the placement of the working portal directly over the desired disk space. The patient is prepped and draped in the standard fashion. A 1 cm skin incision is made at the level of the disk space, and an optical trocar is inserted. The 10 mm laparoscope is then inserted into the optical dissecting trocar and focused on the subcutaneous tissue to allow visualization of the tissue planes. The trocar has two “Winged keel” cutting surfaces that will not penetrate a fascial layer such as the peritoneum unless the trocar is twisted. The three abdominal muscular layers that overlie the peritoneum are penetrated in sequence under direct visualization until the preperitoneal fat is encountered. One or a combination of three different techniques may be used at this point to create a potential space that is superficial to the peritoneum (the retroperitoneal space). The trocar is most frequently used as a dissecting device under direct visualization until the laterally oriented fibers of the psoas major muscle are viewed. Blunt finger dissection can then be used to increase this space. A dissection balloon (Origin, Menlo Park, CA) can be filled with 1 L of air to dissect the retroperitoneal space, more correctly referred to as the retrotransversalis fascia. CO2 insufflation can be used as an alternative in the retroperitoneal cavity up to a pressure of 20 mmHg to create a working space. The longitudinal fibers of the psoas major muscle are then identified. The genitofemoral nerve is usually visualized on the surface of the psoas muscle. FIGURE 27–3 This 49-year-old patient developed progressive scoliosis during a 5-year period with severe back and anterior thigh pain. Preoperative x-rays (A,B) show lateral listhesis at L2–L3 with resulting severe scoliosis. Lateral endoscopic diskectomy and insertion of BAK cage followed by posterior laminectomy for stenosis and internal fixation with pedicle system (C,D) resulted in significant correction of scoliosis and resolution of back and leg complaints. FIGURE 27–4 Cadaver dissection depicts lateral approach to the lumbar spine, with the endoscopic tube penetrating the anterior third of the psoas muscle at the disk space. FIGURE 27–5 This 62-year-old patient presented with degenerative scoliosis at L2–L3 and L3–L4. She was treated with anterior carbon fiber cages through the lateral endoscopic approach, followed by posterior internal fixation using an open pedicle screw system. Following enlargement of the retroperitoneal space, two additional 1 cm incisions are made. The original portal, which is directly orthogonal with the disk space, is used as the working portal for use of the high-speed drill, curettes, and Kerrison and pituitary rongeurs. The second portal is necessary for the 10 mm laparoscope. A third portal is used for retraction of the psoas muscle fibers, and a fourth 10 mm portal is required for suctioning (Fig. 27–6C,D). The dissection is carried in a longitudinal fashion in line with the muscle fibers and through the anterior two thirds of the psoas muscle. When the psoas major muscle is separated during retroperitoneal endoscopy, there is a potential risk of injury to the lumbar plexus or nerve roots. Understanding the relationship between the greater psoas muscle and the lumbar plexus is essential to avoid nerve injury. The genitofemoral nerve branches from the L1 and L2 nerve roots pierces the psoas muscle toward the anterior side of the muscle, and subsequently descends in accordance with the abdominal surface of the psoas major. The level that the genitofemoral nerve passes through the psoas muscle varies from the cranial third of the L3 vertebral body to the caudal third of the L4 vertebral body. In the case of spreading the psoas muscle, it is thought that the more caudal the muscle is spread, the more likely transitory genitofemoral nerve paralysis will occur. For protecting the lumbar plexus or roots and the genitofemoral nerve, the safe zone is in the anterior third of the psoas muscle. The muscle should be split more anteriorly with respect to the vertebral body from the cranial part of L3 and above. Based on anatomical studies, the plexus lies in the posterior half of the vertebral body at L4 and above. The safety zone with respect to the lumbar plexus is at the abdominal edge of the vertebra. The surgical approach should therefore be directed at or above L4 in the anterior half of the vertebral body with respect to a lateral view of the spine. At the L4–L5 disk and below, the plexus can pass anterior to the midaxis of the vertebra as seen on a lateral view. Retraction of the psoas muscle posteriorly should also be avoided because this can trap the exiting root against the base of the pedicle and transverse process. The relatively avascular intervertebral disk space can often be palpated through the anterior portion of the psoas muscle and is exposed first. The midportions of the adjacent vertebral bodies are then exposed. If necessary, the lumbar segmental vessels are ligated and divided. In most cases this is not necessary. FIGURE 27–6 This series of illustrations depicts the lateral approach to L3–L4. (A,B) The patient is in a straight lateral position, where the skin will be marked directly over the disk after fluoroscopy is obtained. (C,D) The initial trocar is placed from the lateral position directly over the disk. Once insufflation is complete, three additional anterior trocars are placed. This is done after using the 10 mm endoscope to dissect the peritoneum toward the midline. (E,F) The final position of a cage with respect to the psoas muscle. It is critical to dissect only the anterior third of the muscle to avoid the femoral plexus. Once the vertebral level is confirmed fluoroscopically, the transversalis fascia, perinephric fascia, and retroperitoneal contents are retracted anteriorly. A Harmonic scalpel (Ethicon Endosurgery, Cincinnati, OH) is used to mark the intervertebral disk space. At this point, it is important for the surgeon to have access to various methods of hemostasis. We most frequently utilize the harmonic scalpel, but we also have bipolar endoscopic electrocautery, Endo-Avitene Microfibrillar Collagen (Humacao, Peurto Rico), and Gelfoam soaked in thrombin available to us. If necessary, the segmental vessels are dissected from the underlying bone and elevated with a right-angled clamp. It is important to use two vascular clips or an endoloop ligature on the high-pressure side of the vessels. The vessels are divided with endoscopic scissors. The 5 mm Harmonic scalpel is also used to ligate segmental vessels. The segmental vessels are ligated and divided in the anterior half of the vertebral body to allow maximal possible collateral circulation to the neural foramen and spinal cord. The disk space is incised using the Harmonic scalpel. Graduated endoscopic curettes and pituitary rongeurs are used to perform a complete diskectomy. The disk space height is restored by using a distraction plug placed from the side. A drill tube is placed over the distraction plug. The position of the distraction plug is monitored with anteroposterior and lateral fluoroscopy. The center of the distraction plug will correspond to the center of the BAK interbody fusion cage.2 It is important to countersink the cage and pack additional bone graft superficial to the cage. The presence of a solid trabecular bone bridge in this location (lateral “Sentinel sign”) allows for confirmation of the arthrodesis after ~3 to 6 months following surgery. The BAK or carbon fiber cages are packed with autogenous iliac graft obtained through a separate incision over the ipsilateral anterior iliac crest (Fig. 27–6E,F). Using the technique described here at L4–L5, it is sometimes necessary to remove part of the iliac crest or place a docking portal through the iliac wing to be orthogonal to the disk space.6,12 A carbon cage can alternatively be inserted at L4–L5 using a portal orthogonal to the L4–L5 space and slightly anterior to the iliac crest, angling obliquely in a slight anterior-to-posterior direction. Patients are repositioned prone for pedicle screw instrumentation when posterior fixation is indicated. Traditional posterior fusion methods are used for scoliosis cases; however, single- or two-level anterior interbody fusions performed through this endoscopic lateral approach may be combined with percutaneous posterior pedicle screw fixation. This combination allows for a minimally invasive approach to 360 degree fusion. The Sextant (Medtronic, Memphis, TN) and Atavi (Endius, Plainville, MA) systems have been used successfully for one- and two-level fusions (Fig. 27–7). FIGURE 27–7 (A,B) This patient underwent endoscopic lateral fusion through the anterior endoscopic approach, followed by posterior two-level Sextant percutaneous fixation. Results and Complications In the McAfee et al5 series, 18 patients underwent a lateral endoscopic retroperitoneal approach for lumbar spinal fusion. There was a short postoperative stay of 2.9 days. All patients obtained a solid arthrodesis, and there were no cases of great vessel injury, retrograde ejaculation, or implant migration. Regan has subsequently compiled a series of 27 consecutive lateral endoscopic transpsoas lumbar spinal fusions. The average operative time was 145 minutes (range: 120 to 170 minutes) for the anterior approach. Average blood loss was 150 ml (range: 50 to 650 ml). Twenty-two of 27 patients (85%) had a good to excellent outcome and would undergo the surgery again. Visual analogue scale (VAS) improved postoperatively by 5.9 for all patients. There were no mortalities, infections, pseudarthroses, implant migrations, or subsidence in this series. There was an incidence of postoperative groin and anterior thigh paresthesias or pain in 8 of 27 patients (30%). These symptoms were self-limiting and resolved within 8 weeks postoperatively. This complication is due to the dissection of the psoas muscle and subsequent postoperative edema/hematoma causing irritation of the genitofemoral nerve. The genitofemoral nerve arises from the L1 and L2 roots. It passes obliquely through the substance of the psoas and emerges from its inner border at a level corresponding to the L3–L4 interspace. It then descends on the surface of the psoas muscle, normally under the cover of the peritoneum, and divides into the genital and femoral branches. The genital branch passes outward on the psoas major and pierces the fascia transversalis, or passes through the internal abdominal ring. It then descends along the back part of the spermatic cord to the scrotum and supplies, in the male, the cremaster muscle. In the female, it accompanies and ends in the round ligament. The femoral branch of the genitofemoral nerve descends on the external iliac artery, sending a few branches to it, and after passing beneath Poupart’s ligament to the thigh, supplies the skin of the anterior aspect of the thigh down about midway between the pelvis and the knee. Two patients underwent conversion from an endoscopic to a mini-open approach. One patient was converted due to adhesions from prior surgery and one patient due to bleeding from a segmental vessel. Vraney et al10 reported that access to the L4–L5 disk space via an endoscopic transperitoneal approach would be readily accessible in only ~33% of patients and in others would require significant dissection. This was based on a review of computer-generated series of abdominal arterial studies and not actual surgical cases or directly observed anatomy. Regan et al7,9 reviewed the results of 58 consecutive patients who underwent laparoscopic ALIF at the L4–L5 level using BAK cages in an attempt to describe variations in the approach used to address anatomical variations in the location of the great vessel bifurcation when approaching this region. The L4–L5 disk space was accessed above the great vessel bifurcation in 30 patients (50%), below the bifurcation in 18 patients (30%), and between the vessels in the remaining 10 patients. Tiusanen et al13 reported a 5.9% incidence of retrograde ejaculation following anterior transabdominal lumbar interbody fusion. There were 12 cases (5%) of retrograde ejaculation that occurred as a complication of laparoscopic BAK interbody fusion and stabilization in the first series of 240 patients submitted to the U.S. Food and Drug Administration.9 The retroperitoneal exposure has not been associated with this postoperative complication.3 The lateral endoscopic retroperitoneal approach for lumbar fusion avoids dissection of the great vessels regardless of the level of bifurcation. Because the autonomic plexus is not dissected, there is a reduced risk of retrograde ejaculation compared with anterior approaches.6 In addition, the lateral decubitus position facilitates exposure of the lumbar spine, as gravity helps in pulling the abdominal contents anteriorly. It is also easier to position a trocar orthogonal to the disk space with a laterally directed interbody fusion device, as opposed to the supine Trendelenburg position required for transperitoneal laparoscopy. Unlike standard anterior approaches, the anterior longitudinal ligament and posterior longitudinal ligament are not violated with the lateral retroperitoneal approach. This confers a significant biomechanical advantage. Moreover, with the transperitoneal approach, if the surgeon reams, taps, or drills too deeply, the spinal canal contents are at risk. With the lateral retroperitoneal approach, these activities are directed toward the contralateral psoas muscle instead of the spinal canal contents.6 In the FDA laparoscopic BAK study,9 the incidence of iatrogenic intraoperative disk herniation in patients undergoing surgery at one level was 2.8% (3 of 25 patients). Overall, for BAK implants inserted via a straight anterior-to-posterior direction, the incidence of reoperation for iatrogenic penetration or for pushing intervertebral disk material into the spinal canal was 2.3%. Posterior instrumentation was used in all patients. Stand-alone anterior lumbar interbody fusion remains controversial, and we feel that providing a posterior tension band reduces the pseudarthrosis rate significantly. We utilize percutaneous pedicle screw fixation on all single-and two-level cases. Scoliosis cases require a traditional posterior approach. This combination provides a minimally invasive approach and allows for 360-degree fusion. There has been a surge in the use of laparoscopic approaches to the lumbar spine for interbody fusion using threaded cages. These techniques are attractive in that they offer the potential for less perioperative pain and morbidity, shorter hospital stays, quicker recovery times, and a faster return to work and the patient’s normal lifestyle. The lateral endoscopic approach to the lumbar spine offers several advantages over traditional techniques. Mobilization of the great vessels is not required, and dissection of the sympathetic plexus is eliminated. REFERENCES 1. Obenchain TG. Laparoscopic lumbar discectomy: case report. J Laparoendosc Surg. 1991;1:145–149. 3. Mayer MH. Mini ALIF: a new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine. 1997;6: 691–700.
27

Endoscopic Lateral Transpsoas Lumbar Spine Fusion

< div class='tao-gold-member'>
Endoscopic Lateral Transpsoas Lumbar Spine Fusion
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








