Video 43.1). Approximately 85% have an epileptogenic focus in the temporal lobe, whereas the remaining 15% are of extratemporal origin, usually frontal.
c. Secondarily generalized (tonic–clonic) seizures. During any focal seizure (simple or complex partial), the epileptic excitation can spread widely to bilateral hemispheric regions, resulting in a generalized tonic–clonic convulsion.
2. Generalized seizures are characterized by generalized involvement of the brain from the outset without consistent focal areas of ictal onset. There are many subtypes.
a. Absence seizures, also known as petit mal seizures, have a dominant feature of brief loss of consciousness with no or minimal motor manifestations (e.g., twitching of the eyelids). During the seizure, the EEG shows 3-Hz generalized spike–wave discharges. Absence seizures are often divided into typical and atypical absences.
b. Myoclonic seizures are brief jerks involving part of the body or the entire body (![]() Video 43.2).
Video 43.2).
c. Clonic seizures are rhythmic twitching of the body.
d. Tonic seizures are brief attacks of stiffness in part of the body or the entire body.
e. Atonic seizures are losses of posture with resultant drop attacks.
f. Tonic–clonic seizures are generalized convulsions or grand mal seizures. It is important to emphasize that some of these are generalized from the outset and some are secondarily generalized, that is, they start as focal seizures and then become generalized. The second type is the most common among adults. The presence of an aura, focal manifestations during the seizure, and postictal focal deficits favor a secondarily generalized tonic–clonic seizure.
Confusion can arise in differentiating absence seizures and complex partial seizures. Both can present with a brief loss of awareness or altered responsiveness, and in both there may be automatic activities of various kinds. Diagnosis is aided by EEG findings (generalized spike–wave discharges in absence seizures and focal epileptiform abnormalities in complex partial seizures). Correct diagnosis is critical for instituting proper antiepileptic drug (AED) therapy.
B. Classification of epilepsy or epileptic syndromes (1989). Classifying the seizure type, although useful, is of limited value because seizures usually appear as part of a cluster of other symptoms and signs that include etiologic factor, site of seizure onset, age, precipitating factors, response to medication, and prognosis. Hence, the ultimate goal is to diagnose an epileptic syndrome. This is very important. It helps to choose the appropriate AEDs to control seizures and to avoid using an AED, which may not only be ineffective but may even exacerbate seizures.
1. Localization-related (focal or partial) epilepsy or epileptic syndromes are disorders in which a localized origin of the seizures can be established. The patient has focal or secondarily generalized tonic–clonic seizures. EEG shows focal epileptiform discharges overlying the epileptogenic focus.
a. Most localization-related epilepsies are acquired or symptomatic. Temporal lobe epilepsy is the common localization-related epilepsy among adults, and it is often associated with mesial temporal sclerosis on the magnetic resonance imaging (MRI) scan.
b. There are age-related idiopathic or primary localization-related epileptic syndromes. The best known is benign rolandic epilepsy of childhood.
2. Generalized epilepsy or epileptic syndromes are disorders that involve one or more types of generalized seizures. EEG shows generalized epileptiform abnormalities.
a. Primary (idiopathic) generalized epilepsy (PGE) is characterized by generalized seizures without any identifiable etiologic factor. Genetic factors predominate. EEG shows generalized spike–wave or polyspike–wave discharges with a normal background activity. Common syndromes include childhood absence epilepsy, juvenile myoclonic epilepsy (JME), juvenile absence epilepsy, and tonic–clonic seizures occurring often in the early morning (“awakening” grand mal).
b. Secondary (symptomatic) generalized epilepsy is characterized by various types of generalized seizures resulting from acquired cerebral diseases (e.g., seizures secondary to ischemic–hypoxic encephalopathy or following severe cerebral trauma or intracranial infection) or from inborn errors of metabolism (e.g., lipidosis and progressive myoclonus epilepsy). EEG shows generalized, irregular spike–wave, 2.5 Hz or less in frequency with an abnormally slow background activity. Patients usually have varying degrees of cognitive and neurologic deficits, and the seizures are often drug resistant. Within this category are three commonly recognized age-related syndromes—West’s syndrome (infancy) and Lennox–Gastaut syndrome (childhood) and severe epilepsy with independent multifocal spike discharges occurring in childhood and adults.
C. Newer ILAE classification of epileptic seizures and epilepsies (2010).
1. The 2010 classification of seizures is still very similar to the previous one of 1981 and continues to separate epileptic seizures into generalized and focal seizures, but emphasizing their origin in neural networks rather than in discrete anatomical regions. The generalized seizures arise in some cortical area with rapid spread to bilaterally distributed neural networks including cortical and subcortical structures, but not necessarily the entire cerebral cortex. The focal seizures are categorized as those arising in neural networks in one hemisphere remaining discretely localized before spreading more widely. Epileptic spasms have been included under the category without a clear-cut focal or generalized onset.
2. In the 2010 classification, the focal-onset epileptic seizures are not subdivided into simple partial or complex partial seizures as in 1981 classification because alteration of awareness is often difficult to ascertain and that the pathophysiologic mechanism may not be distinctive in the two subtypes.
3. For the epileptic seizures of generalized onset, there is very little change compared to the 1981 classification. Myoclonic seizures are further refined into myoclonic–atonic (jerk followed by decreased muscle tone) and myoclonic–tonic (jerk followed by increased muscle tone). Absences with eyelid myoclonia and myoclonic absences have been added to the typical and atypical absences.
4. The original ILAE classification of epilepsies and epilepsy syndrome (1989) was based on the dichotomy of focal- versus generalized-onset seizures and identifiable etiology (symptomatic with known identifiable etiology and idiopathic without identifiable cause) resulting into four subcategories: symptomatic focal (localization-related) epilepsies, idiopathic focal (localization-related) epilepsies, idiopathic (primary) generalized epilepsies, and symptomatic (secondary) generalized epilepsies. In the 2010 classification, these terms have been largely abandoned. The dividing line between idiopathic and symptomatic generalized epilepsies is often difficult to define with significant overlap. The new proposal recommends classifying epilepsies according to distinctive electroclinical syndrome and identifiable causation. The epilepsies are broadly divided into:
a. Age-related electroclinical syndrome (neonatal period, infancy, childhood, adolescence to adult)
b. Surgical syndrome (temporal lobe epilepsy with hippocampal sclerosis, Rasmussen syndrome, gelastic seizures with hypothalamic hamartoma)
c. Epilepsy with structural-metabolic causes
d. Epilepsies of unknown cause
Even though the newer classification of epilepsies and epilepsy syndrome is gaining some acceptance, the older classification still remains strongly entrenched into the vocabulary of epilepsy community. The author has, therefore, decided to maintain it in this chapter for the time being until the newer classification gets more widely accepted.
EVALUATION
It is essential to establish that the spells or episodes are indeed epileptic seizures. Nonepileptic physiologic disorders that result in transient, reversible alterations of behavior or function, such as syncope, migraine, breath-holding spells, anxiety episodes, transient ischemic attacks, hypoglycemic episodes, and narcoleptic–cataplectic attacks, must be differentiated from epileptic seizures. Moreover, there are nonepileptic psychogenic seizures or pseudoseizures that are conversion reactions characterized by episodes of motor activity and lack of awareness but not associated with ictal EEG patterns and without an underlying physiologic basis.
A. A history of the episodes, obtained not only from the patient but also from one or more observers, is perhaps the most essential element in making the diagnosis of epileptic seizures and differentiating them from nonepileptic disorders.
B. Physical and neurologic examinations can help detect the underlying cause of the brain disorder responsible for the epilepsy by uncovering evidence of a focal cerebral lesion or another neurologic disorder, such as tuberous sclerosis or neurofibromatosis.
C. Neuroimaging. Compared to computed tomography of the head with and without contrast, MRI of the brain is the imaging procedure of choice. It is particularly sensitive in detecting hamartoma, cavernous malformation, low-grade glioma and mesial temporal sclerosis.
D. EEG is the most informative test for confirming epileptic nature of the episodes, classification of the seizure type, and even the epileptic syndrome. It also aids in initiation, selection, and discontinuation of antiepileptic therapy. Not all patients with epilepsy have interictal epileptiform abnormalities; approximately 50% have such abnormalities in a routine awake-and-asleep EEG study that includes hyperventilation and intermittent photic stimulation. The yield increases with repeated EEG studies with sleep deprivation and extra recording electrodes. On the other hand, 1% to 2% of healthy persons without clinical seizures have epileptiform abnormalities in EEG studies. Hence, an interictal EEG alone can neither prove nor exclude a diagnosis of epilepsy. Similarly, the presence of interictal epileptiform EEG abnormalities does not automatically warrant AED therapy, and the absence of such abnormalities is not sufficient grounds for discontinuing AED treatment.
E. 24-hour video EEG or prolonged EEG. This is indicated to detect epileptiform abnormalities in those patients who had previously a normal routine EEG (awake and asleep) and in whom the epileptic nature of the episodes needs to be established before starting or changing antiepileptic medication.
F. Prolonged video EEG monitoring. Patients with drug-resistant epilepsy or poorly characterized episodes may need prolonged monitoring that consists of simultaneous monitoring of the patient’s behavior and EEG to provide detailed clinical and EEG correlation of episodic events. This is an expensive and time-consuming technique and is thus left to the discretion of a consulting neurologist specialized in epilepsy. Only 5% to 10% of patients believed to have epilepsy need this technique to characterize and classify the epileptic episodes. Video EEG is mandatory in the presurgical evaluation of patients to document epileptic seizures prior to surgical resection to treat epilepsy, and even before placing a patient on vagus nerve stimulation (VNS). It is also very helpful in patients who have frequent episodes that are suspected to be of the nonepileptic psychogenic type.
G. Home video recording. With the universal availability of “smart phones” the relatives are encouraged to record one or more of the patient’s events. This cheapest monitoring can often provide very critical information regarding the nature of the episodes even without the benefit of concomitant EEG as in the formal video EEG monitoring.
GENERAL PRINCIPLES IN TREATING PATIENTS WITH EPILEPSY
A. AED therapy should be initiated only when the diagnosis of epileptic seizures is well established. If the patient’s episodes are yet to be clearly defined and there is reasonable doubt of their being epileptic in nature, it is prudent to wait until the diagnosis of epilepsy can be confirmed.
B. Monotherapy is preferable to the use of several drugs because it has fewer toxic side effects, less likelihood of drug interactions, and better compliance. The chosen AED (see Table 43.1) should be slowly increased until seizures are controlled or until clinical signs of toxicity develop. If seizures are not adequately controlled at the maximum tolerable dosage, a second AED is slowly introduced. After the second drug attains therapeutic levels, the first drug is gradually withdrawn. Monotherapy adequately controls new-onset epilepsy in about two-thirds of the patients.
C. Polytherapy with a combination of two AEDs becomes necessary only if monotherapy trial of two or more first-line AEDs has been unsuccessful. When using two AEDs, select those with different mechanism of action. Avoid using more than two AEDs simultaneously. If a combination of two AEDs in the treatment of a compliant patient with blood levels in the therapeutic range fails to provide adequate control of epileptic seizures, referral to an epileptologist or comprehensive epilepsy center is indicated for further evaluation and management.
D. AEDs with sedative or hypnotic side effects (e.g., phenobarbital, primidone, and clonazepam) need to be avoided unless first-choice AED does not work. A patient on polytherapy that includes one of the aforementioned sedative AEDs is best served by a gradual withdrawal of the sedative AED while the dosage of the other AED is maximized. Discontinuation of sedative–hypnotic AEDs is followed not only by a reduction in side effects but also by better control of seizures in many instances.
E. Simplify drug schedules. With few exceptions, most AEDs have long elimination half-lives (see Table 43.3), and thus can be prescribed in one or two divided daily doses.
F. Adherence must be emphasized. Medication is best taken at the time of meals for easy remembrance. For most AEDs, an occasional missed dose can be made up by taking an additional dose within the same 24-hour period. It is also convenient for the patient to put the medication in a plastic pillbox with divided compartments and to make sure at bedtime that the entire day’s medication has been taken.
G. Advise the patient to maintain a seizure diary and bring it at every office visit to assist in evaluating the effectiveness of the therapy.
H. Emphasize to the patient the need for constant medical follow-up care. Once AED therapy is well established and the seizures have been brought under satisfactory control, the patient may be followed every 6 to 12 months. During the follow-up visits, evaluate the patient for evidence of drug toxicity or development of a progressive neurologic disorder. Complete blood count (CBC), liver function tests, and serum electrolytes may need to be performed every 6 to 12 months to detect untoward effects of AEDs on the bone marrow and liver in patients receiving valproate, carbamazepine, and phenytoin. However, routine blood testing at periodic intervals is a controversial issue because serious side effects are rare and, when they occur, they do so over a short period to be detected by periodic monitoring.
I. A patient who achieves good control with drug therapy may have a “breakthrough” seizure during periods of physical or mental stress, sleep deprivation, or febrile illness. Appropriate management of such precipitants rather than increases in dose or changes in the AED is indicated.
J. Generic substitution for brand-name AEDs can reduce the cost of medication, but the bioavailabilities of generic and proprietary AEDs are not the same. Generic preparations are required by the US Food and Drug Administration (FDA) to provide bioavailabilities within ±20% of those of the corresponding proprietary formulations, but some patients may be sufficiently sensitive to these fluctuations so that replacing one with the other may lead to either loss of seizure control or signs of neurotoxicity. This problem applies primarily to phenytoin and carbamazepine. It is probably prudent to continue brand-name AED if the patient is seizure free on that formulation. If changed to generic AED the patient needs to be warned of the possibility of breakthrough seizures or drug toxicity. When a generic AED is used, the formulation by the same manufacturer is preferred.
K. Therapeutic drug levels are rough guides to the ranges that provide best seizure control while avoiding dose-related side effects. The blood should be drawn preferably before the morning dose of AEDs so as to obtain the lowest (trough) levels. The blood levels are not to be followed rigidly for a given patient. Some patients may attain complete seizure control at low “therapeutic” levels, and increasing the dose to attain idealized levels is not indicated. On the other hand, there are patients who need higher than “therapeutic” levels for control of their seizures and tolerate such levels without significant untoward side effects. AED blood levels are indicated under the following circumstances:
1. To determine the baseline plasma dose level
2. When the patient is believed to be noncompliant
3. When the patient does not respond adequately to the usual dosage of an AED
4. When symptoms and signs of clinical toxicity are suspected
5. When there is a question of drug interaction
6. To establish the correct dosage for a pregnant patient or a patient with diseases affecting the pharmacokinetics of the AEDs (hepatic, renal, or gastrointestinal disorders).
Usually total serum levels of AEDs are obtained. When metabolism of AEDs may be altered or serum protein levels are likely to be low (e.g., hepatic or renal disorders and pregnancy), free levels of highly protein-bound AEDs may become necessary.
L. Emphasize the need to regularize the time and duration of sleep, because sleep deprivation tends to potentiate seizures.
M. Concomitant use of other drugs. Alcohol in any form is best avoided or used in small amounts (e.g., one drink) because of possible interactions with most AEDs. Be aware of drugs that lower the seizure threshold (e.g., tricyclic antidepressants, Welbutrin, and phenothiazines) or those that can cause drug interactions (increasing or decreasing the levels of AEDs); they should be used with caution. Some AEDs affect the elimination kinetics of many drugs metabolized in the liver (e.g., birth control pills, corticosteroids, anticoagulants, and cyclosporine), necessitating proper dose adjustment of these comedications.
N. Encourage the patient to make some life adjustments necessary to lead a normal life as much as possible. Moderate exercise needs to be encouraged; it does not affect seizure frequency. Participation in highly competitive sports increases the risk of physical injury. Individualize instructions to the patient by considering the risk of a particular sport against the patient’s aspirations. Swimming may be permitted under supervision for a patient with good control of seizures. Bathing in a bathtub is to be avoided; instead, a shower is recommended.
O. Most adults who have epilepsy are able to maintain competitive employment and should be encouraged to do so. This improves their self-esteem and their acceptance in the mainstream of society. There are, however, some realistic limitations. Occupations, such as working with heavy machines, working above ground level, working close to water or fire, driving trucks or buses, and flying planes, may be off limits for reasons of personal and public safety.
P. Educate the family members or caregivers regarding proper care of the patient when a seizure occurs. During a grand mal seizure, the patient should be helped to lie on the ground, a bed, or a couch and should be turned on one side to avoid aspiration. An object such as a spoon or a finger should never be thrust into the patient’s mouth. Pushing a hard object into the mouth often results in broken teeth. The patient must be closely watched and the sequence of events carefully observed during a seizure, which can help determine the type of seizure.
Q. Hospitalization. For a patient with a known history of seizures, an isolated self-limiting seizure does not constitute a need to call for an ambulance and to rush the patient to an emergency department. However, if the seizure lasts longer than 5 minutes or if the patient has repeated seizures without regaining consciousness between them, prompt transfer to a nearby hospital becomes essential. Medical attention must also be sought if the patient had a fall and sustained bodily injury.
R. Driving. Most states have laws denying driving privileges to patients with uncontrolled epilepsy but permit driving once the seizures have been brought under control with AEDs. In a few states, doctors are required to report cases of epilepsy. The period of time that the patient must remain seizure-free before being permitted to drive varies from 3 months to 2 years, depending on the state. Rare patients who have only nocturnal seizures or who have only simple partial seizures (no loss of consciousness) may be exempted from driving restrictions. Reinstitution of driving privileges may require reapplication, a letter from the treating physician, or a determination made by a state-appointed board. Some states require the treating physician to certify at regular intervals that the patient has continued to remain seizure-free before reissuing the driving permit. Because the laws regarding driving vary widely among different states and are frequently changing, physicians are best advised to obtain their current state registration. In general, patients with frequent seizures with altered consciousness must be advised to refrain from driving until seizures can be satisfactorily controlled. That the patient has been properly advised must be documented in the patient’s record. There is no consensus as to how long the patient should be advised not to drive in the case of a breakthrough seizure after being seizure-free for a long period. If such a seizure follows a known precipitant such as infection, mental or physical stress, prolonged sleep deprivation, or poor compliance, observation for at least 3 to 6 months is required before driving is permitted again.
ANTIEPILEPTIC DRUGS
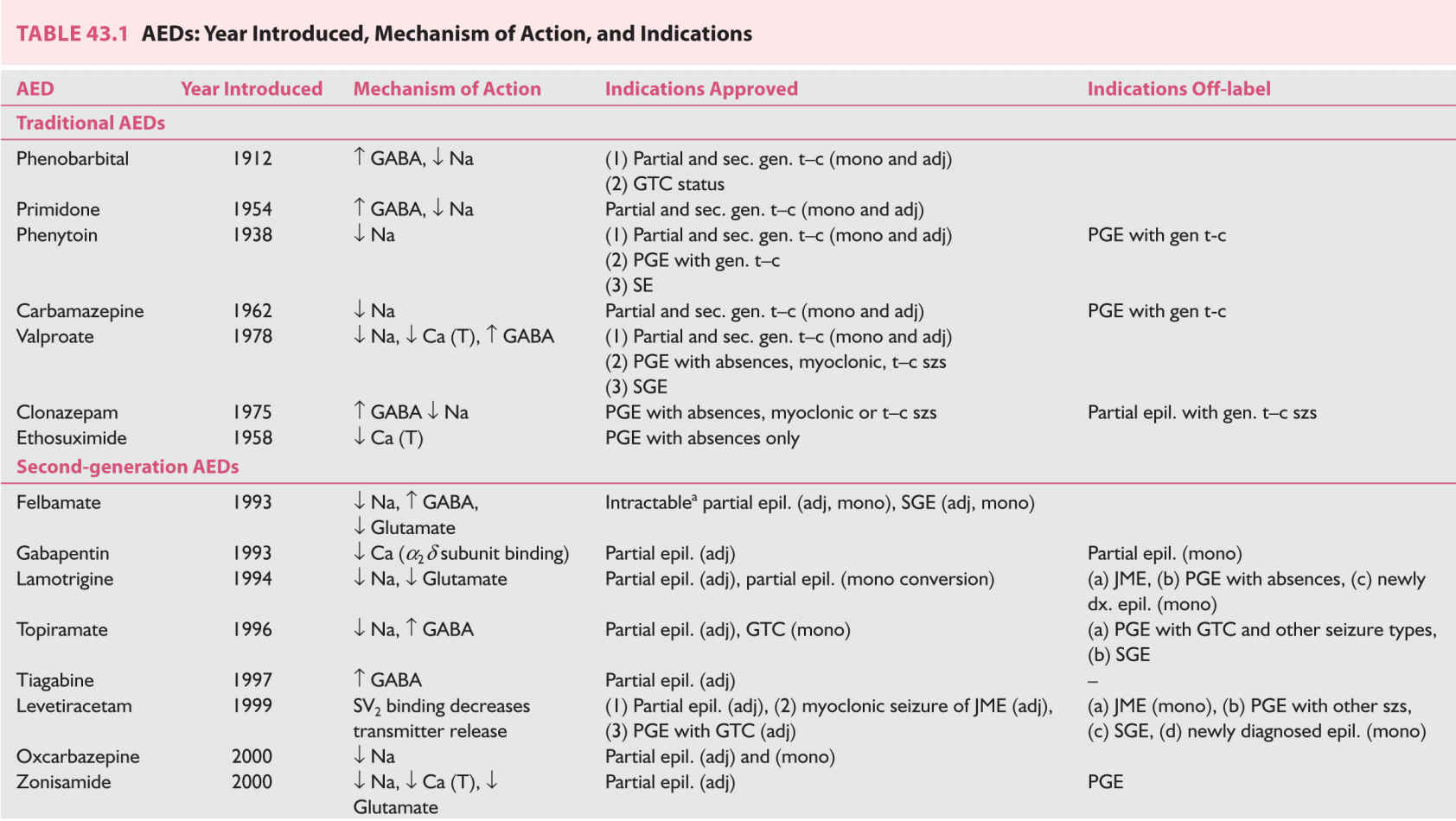
A. Mechanism of action, and efficacy of AEDs. Table 43.1 lists the AEDs presently available in the United States. The table summarizes the mechanism of action, year of introduction in the treatment of epilepsy, and FDA-approved as well as off-label utility of these AEDs.
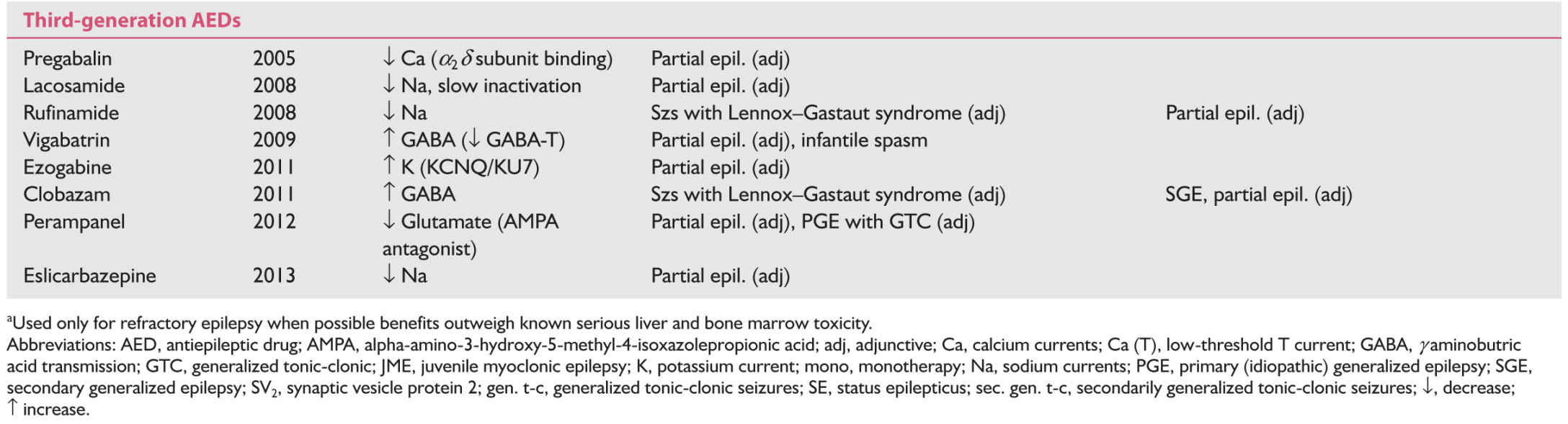
According to the duration of availability, the AEDs can be divided into first-generation or traditional AEDs, which were available before 1993; second-generation AEDs, which became available between 1993 and 2004; and third-generation AEDs, which became available in the United States in or after 2005. The second- and third-generation AEDs are often grouped as Newer AEDs but sufficient clinical experience, side-effect profile, and teratogenic data are available only for the second-generation AEDs.
An ideal AED should be effective against multiple seizure types, effective against seizures resistant to traditional AEDs, have low neurologic and systemic toxicity, and should have favorable pharmacokinetics: complete oral absorption, minimal binding to plasma protein, primarily renal elimination, long elimination half-life, linear kinetics, and no enzyme induction or inhibition. Newer AEDs have substantial advantages over traditional AEDs including absence of side effects, none or fewer drug interactions, and favorable pharmacokinetics. Therefore, the second-generation AEDs have largely replaced the traditional AEDs in the treatment of epilepsy, especially in the developed countries. However, there is no evidence that any new AED has better efficacy compared with carbamezapine, phenytoin, or valproic acid in well-controlled trials of recent-onset epilepsy.
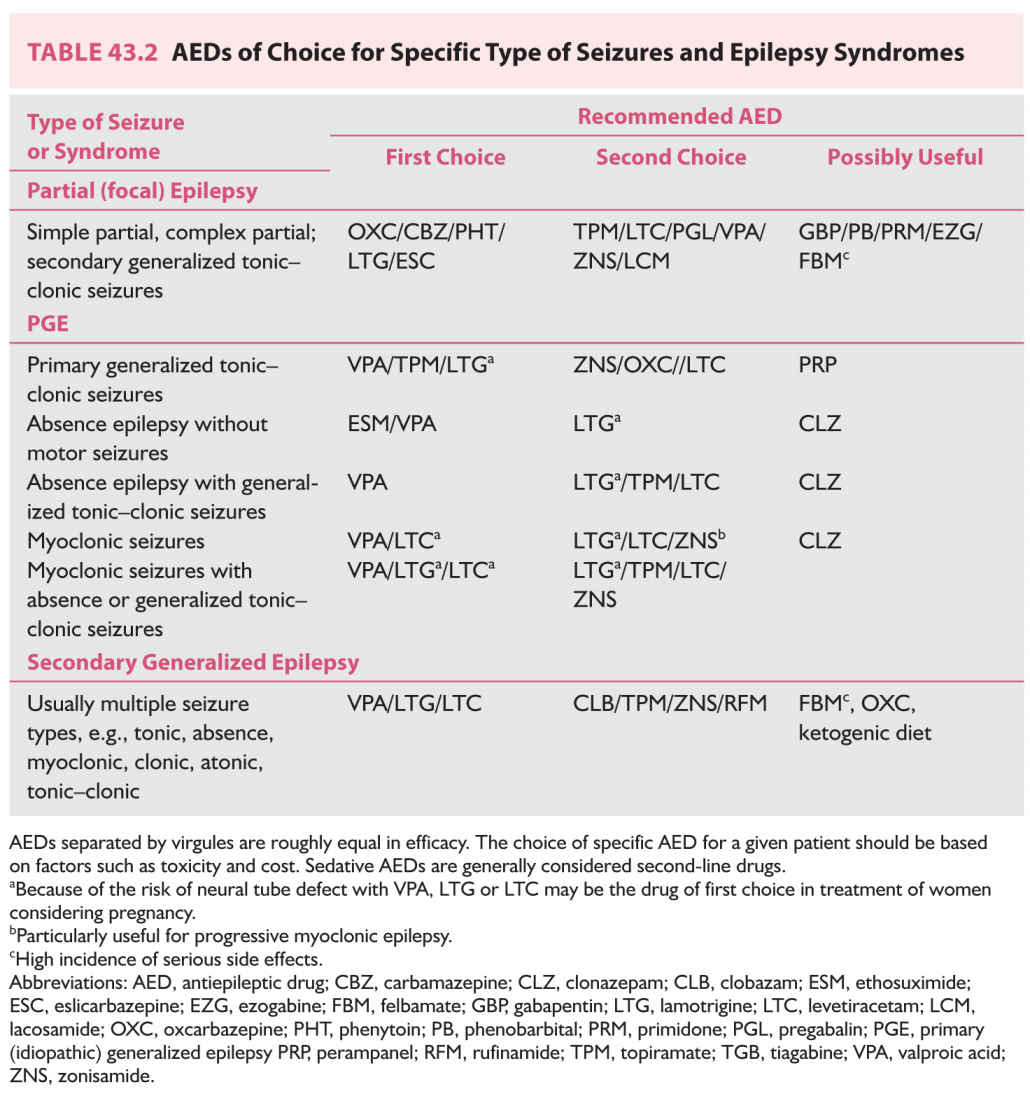
B. Proper selection of AED. Table 43.2 lists AEDs effective for managing various forms of epilepsy and epileptic syndromes.
1. Symptomatic partial (localization-related) epilepsy. Of the traditional AEDs, carbamazepine, phenytoin, primidone, and phenobarbital are effective in the treatment of partial epilepsy but carbamazepine and phenytoin are usually better tolerated than primidone and phenobarbital. Carbamazepine and phenytoin have been the first-line “traditional” AEDs for several decades before the introduction of the newer AEDs. They are used progressively less except in the developing countries. Phenytoin is relatively inexpensive, can be titrated rapidly in 2 to 3 days, is better tolerated in the initial period of therapy, and can be given in one to two divided daily doses. However, it has a high incidence of chronic dysmorphic side effects, such as hirsutism, coarsening of facial features, and acneiform eruptions, which makes its use rather unacceptable in women. Its nonlinear kinetics makes the dose adjustment difficult during maintenance therapy. Carbamazepine has no dysmorphic effects; hence, it is better accepted by adolescent and young adult female patients. Its short half-life usually necessitates using it in three or four divided doses, but sustained-release preparations are now available and given in two daily doses. It needs to be slowly titrated over 3- to 4-week period because of autoinduction. Valproate is the only broad-spectrum AED of first generation, effective both in partial or generalized epilepsies. It is probably less effective than phenytoin or carbamazepine for partial epilepsy. All the three require periodic blood monitoring for bone marrow and liver functions. Most of the newer AEDs are approved by the FDA for adjunctive therapy in the treatment of partial epilepsy refractory to traditional AEDs with the exception are oxcarbazepine. Oxcarbazepine, which has lesser side effects, rapid titration, and no requirement for blood monitoring, has emerged a more favored newer AED alternative to carbamazepine since its approval by the FDA for monotherapy in adults with focal epilepsy. If monotherapy with oxcarbazepine fails to achieve satisfactory control, an adjunctive therapy with another second-generation AED should be strongly considered by adding levetiracetam, lamotrigine, topiramate, or zonisamide to oxcarbazepine. These four AEDs are also broad spectrum in efficacy. Levetiracetam is gaining more extensive use not only as adjunctive therapy but also as monotherapy (off label in the United States) because of rapid titration, no need for blood monitoring, absent hepatic metabolism, and lack of interaction with other drugs. One-fourth may develop anxiety and other behavioral side effects necessitating its discontinuation. Lamotrigine is another widely used newer AED in partial epilepsy, especially in women of childbearing age because of its very low teratogenic potential. However, it requires slow titration because of skin hypersensitivity. It is approved by FDA for monotherapy conversion in addition to adjunctive therapy. Although it does not affect metabolism of oral contraceptive drugs, the latter significantly decrease lamotrigine levels. Adjunctive therapy with topiramate (multiple mechanisms of action against epileptic process) is particularly helpful in those patients who have comorbidity of migraine and/or obesity because of its efficacy in migraine and its weight-loss effect. However, it does have significant cognitive side effects, risk for kidney stones, and relatively higher incidence of teratogenesis. Of the third-generation AEDs, lacosamide is being used more frequently as adjunct therapy in partial-onset seizures if lamotrigine, levetiracetam, or oxcarbazepine has not succeeded in satisfactory seizure control. Combination therapy with lacosamide is more likely to produce neurotoxic side effects. Esclicarbazepine, with a similar molecule as oxcarbazepine, is recently approved as monotherapy for the treatment of focal-onset epilepsy. There is yet very little clinical experience with this AED but it is reported to have a better side-effect profile than oxcarbazepine. Felbamate, a broad-spectrum AED, is very effective in partial epilepsy and has little or no sedative side effect. It is, however, recommended as a last resort because of its hepatic and bone marrow toxicity. One would need a signed consent form and periodic blood testing.
Even with adequate AED therapy, only 40% to 60% of patients with symptomatic partial epilepsy (the most common type of epilepsy among adults) attain full control of seizures. In one study of new-onset epileptic seizures, initial monotherapy was effective in 47% of patients. Changing to a second drug controlled another 13% of patients. Use of a third AED or combined therapy with two or more AEDs controlled just 4% of additional patients. Most experts believe that, if an adequate trial with two AEDs either as monotherapy or combined therapy fails, the patient has medically refractory epilepsy. Such a patient is better managed at a comprehensive epilepsy center.
2. PGE. Patients with PGE have either absence, myoclonic, or tonic–clonic seizures. Most, however, have more than one type of seizure, although one type may dominate. Depending on seizure type, several epileptic syndromes are identified under the heading PGE. The best example is JME, which is characterized by myoclonic seizures in the early hours after waking, but most patients also have occasional tonic–clonic seizures. Less often, even absence seizures may occur. Other syndromes include primary tonic–clonic seizures (contrasted to secondarily generalized tonic–clonic seizures, which are part of focal epilepsy), which commonly occur in the morning hours and hence are called “awakening” grand mal seizures. Absence seizures as the dominant manifestation of PGE commonly occur in childhood (childhood absence epilepsy), but in rare cases start in adolescence or early adulthood (juvenile absence epilepsy).
Patients with PGE who have absence seizures only can be treated with either ethosuximide or valproic acid because both are equally effective in controlling absences. Ethosuximide has narrow spectrum of efficacy being specific for absences only and not effective against myoclonic and/or tonic–clonic seizures. Valproic acid is the drug of choice for PGE in patients with multiple seizure types, including the syndrome of JME, primary grand mal seizures, or combined absence–grand mal epilepsy. The advantage of valproic acid is that it is effective against all seizure types comprising PGE. Because of the high incidence of fetal malformations, polycystic ovarian syndrome (PCOS), and weight gain, the use of valproic acid in women with PGE needs to be restricted because recent well-designed studies have demonstrated effectiveness of the newer AEDs, several of which are broad spectrum in their efficacy. Lamotrigine is effective against absences, has low teratogenicity, and is a weight-neutral AED. It is a good alternative for treating absences associated with childhood and juvenile absence epilepsy. Slow titration and dermatological hypersensitivity are some of the undesirable features. It is also found to be effective in JME, although it can occasionally exacerbate myoclonic seizures. Levetiracetam is effective in all seizure types accompanying PGE and particularly tonic–clonic and myoclonic seizures. Topiramate is effective in PGE manifesting with generalized tonic–clonic seizures and approved by the FDA for this indication. However, its side-effect profile (cognitive impairment, renal stones, glaucoma, and teratogenesis) limits its utility. Perampanel is also found to be effective and approved for tonic–clonic seizures of PGE. It may be an alternative in primary generalized tonic–clonic seizures, which have failed to respond to conventional AEDs. Zonisamide is probably effective, also, against all seizure types of PGE, but has more sedative side effects than lamotrigine and levetiracetam. Clonazepam is an effective AED for myoclonic and absence seizures but has certain disadvantages. It has a high incidence of sedative and cognitive side effects, and patients develop a tolerance to its antiepileptic potency after several months of therapy. It may be used as a bedtime medication in those who have myoclonic or generalized tonic–clonic seizures consistently during sleep or in the morning on awakening.
Although most patients with PGE can be well controlled with appropriate AEDs, some 15% are medically refractory. Some are pseudorefractory because they are on wrong AEDs. Sodium-channel blocking and gamma-aminobutric acid (GABA)-enhancing AEDs have been recognized to exacerbate certain seizure types in patients with PGE. Carbamazepine, oxcarbazepine, phenytoin, vigabatrin, tiagabine, gabapentin, and pregabalin commonly exacerbate absence seizures, myoclonic jerks, or both, and should not be used in the treatment of PGE. It is, therefore, critical that the patient needs to have the syndromic diagnosis of PGE established even though the precise subtype of PGE may not be identified. This will prevent iatrogenic exacerbation of seizures. In those patients where the syndromic diagnosis is yet not established, one should consider using broad-spectrum AEDs such as lamotrigine, levetiracetam, topiramate, or valproate.
In refractory cases of PGE, combination therapy using Depakote with either levetiracetam, lamotrigine (be aware of markedly decreased elimination of lamotrigine by valproic acid), topiramate, or zonisamide may be more effective. Combination of valproic acid and ethosuximide may control absences more effectively than either drug alone. Use of valproic acid in a woman of childbearing age should be considered as a last resort because of its high potential for teratogenesis. VNS is used, albeit rarely, in medically refractory PGE.
3. Secondary (symptomatic) generalized epilepsy, which is secondary to multifocal or diffuse cerebral disorders (static or progressive), occurs mostly among children and less often among adults. Patients have multiple seizure types, including atypical absence seizures, myoclonic seizures, tonic seizures, tonic–clonic seizures, and drop attacks.
In general, response to any AED is poor, with only 20% to 40% of patients attaining acceptable seizure control. Such patients commonly end up being treated with polypharmacy, which not only fails to provide better seizure control than do one or two AEDs but may even exacerbate certain types of seizures (absence seizures, myoclonic seizures, and drop attacks).
Valproic acid, being a broad-spectrum AED, is of first choice for secondarily generalized epilepsy and may be started as monotherapy. However, most patients need an addition of newer AEDs. Of these, lamotrigine, levetiracetam, topiramate, and zonisamide have been found useful in clinical trials. Felbamate, rufinamide, and clobazam have been approved by FDA for the control of seizures associated with secondary generalized epilepsy of the Lennox–Gastaut type but felbamate has potentially serious hepatic and bone marrow toxicity requiring periodic blood testing. Clobazam is being used progressively more since the approval by FDA in combination with valproic acid, lamotrigine, or levetiracetam to obtain a better seizure control. When drug combinations are prescribed, appropriate dosages should be used to avoid sedation, which tends to exacerbate minor seizures as well as precipitate statuses in such patients. VNS is another modality of treatment commonly used in this population.
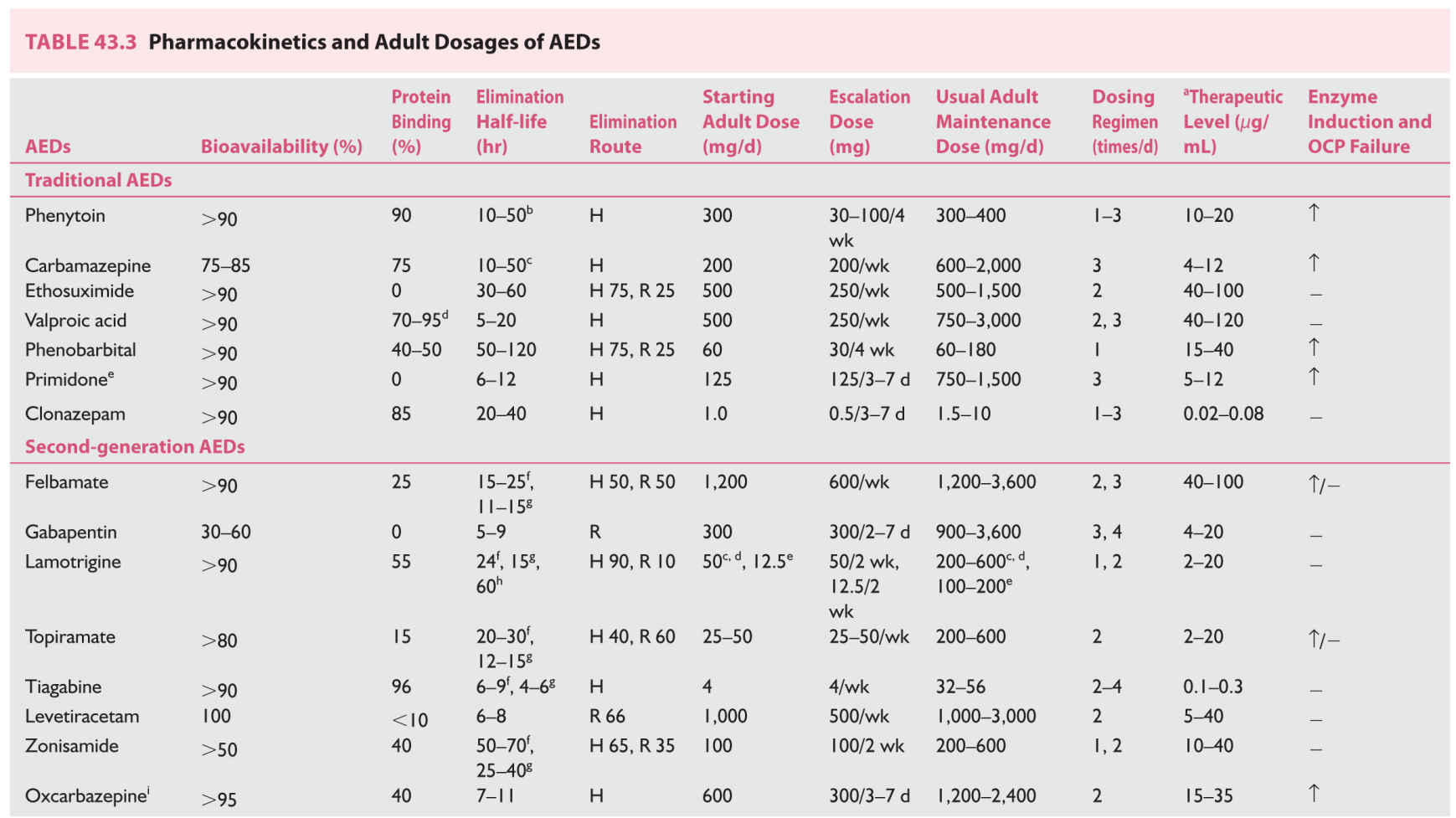
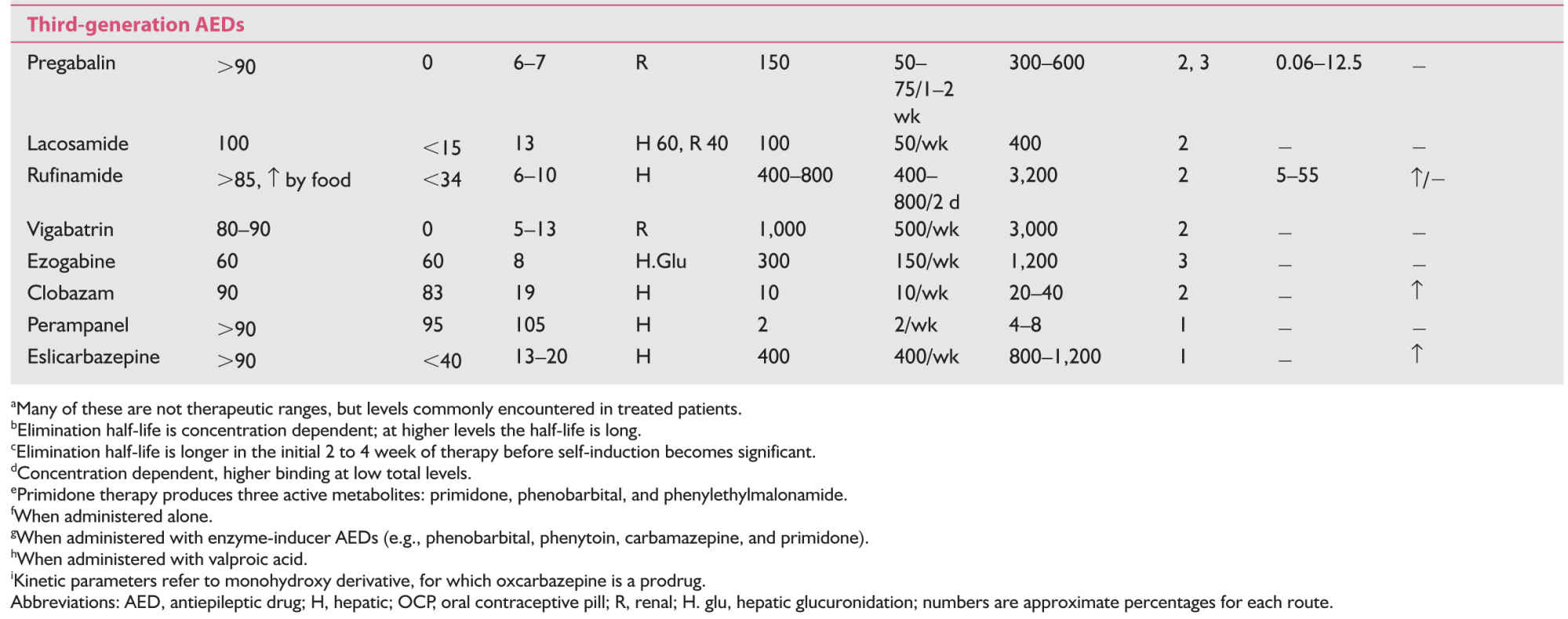
C. Pharmacokinetics of AEDs. These are summarized in Table 43.3.
1. Traditional (first-generation) AEDs.
a. Most of the traditional AEDs are eliminated primarily by hepatic metabolism, through microsomal cytochrome P-450 enzymes, an undesirable pharmacokinetic characteristic.
Because of several potential drug interactions, these enzyme-inducing AEDs lower hormonal levels including oral contraceptives, warfarin, steroids, tricyclics, cyclosporine, digitalis, and antipsychotics.
b. Carbamazepine is notorious in causing autoinduction, so that a smaller dose at the start is associated with blood levels, which are achievable only by two to three times that dose after a month or so. On the other hand, competitive enzymatic inhibition increases the levels of these AEDs causing clinical toxicity. Erythromycin markedly inhibits metabolism of carbamazepine. Cimetidine and propoxyphene have similar but lesser effects. These drugs, as well as grapefruit juice, may result in rise of carbamazepine to toxic levels.
c. Valproic acid is a strong competitive inhibitor of certain hepatic enzymes, increasing the levels of many drugs including phenobarbital, lorazepam, lamotrigine, gonadal, and adrenal androgens.
d. Phenytoin and valproic acid have extensive plasma protein binding (80% to 95%), another undesirable pharmacokinetic property. Valproic acid, in addition, has nonlinear binding; at higher levels, the proportion of protein-bound drug is less, and there is a disproportionately high level of free or unbound drug. It is the free fraction that relates to drug efficacy and clinical toxicity.
e. Another troubling pharmacokinetic characteristic of phenytoin is its nonlinear elimination resulting from saturation of hepatic microsomal P-450 enzymes. Small increments (by 30 mg capsules) need to be made within the therapeutic window of 10 to 20 mg/ mL. Giving a 100 mg capsule to a patient with a blood level of 15 mg/mL on a dose of 300 mg/day may lead to levels close to 30 mg/mL with the risk of clinical toxicity. Similarly, higher serum levels require a relatively small decrease in the dose to optimize the level.
2. Newer AEDs.
They have much better pharmacokinetic properties compared to those of traditional AEDs.
a. Except zonisamide, gabapentin (dose-related absorption), and ezogabine, all the newer AEDs have 90% or more bioavailability with oral intake.
b. Hepatic metabolism is significant only with zonisamide and tiagabine, but they do not cause significant enzymatic induction.
c. Lamotrigine, oxcarbazepine, eslicarbazepine, and ezogabine although metabolized in the liver do not involve cytochrome P-450 enzyme system (phase I reaction). They undergo extensive metabolism by glucuronidation (phase II reaction).
d. Topiramate, levetirecetam, gabapentin, pregabalin, and vigabatrin are eliminated either completely or largely by renal route without undergoing significant hepatic metabolism.
e. Oxcarbazepine, eslicarbazepine, clobazam, and topiramate do induce the metabolism of oral contraceptives, but the effect of topiramate is significant only with doses over 400 mg/day.
f. Most of the newer AEDs, with the exception of tiagabine, clobazam, and perampanel have either no or clinically insignificant binding to the plasma proteins.
g. All of the newer AEDs have linear kinetics and no autoinduction.
h. Significant drug-to-drug interactions are absent with the use of gabapentin, pregabalin, levetiracetam, zonisamide, and lacosamide.
i. Most relevant interaction is between valproic acid and lamotrigine; valproic acid decreases the metabolic elimination of lamotrigine, increasing its half-life three to four times. In patients on valproic acid, lamotrigine needs to be started at a very low dose, titrated up very slowly, and the maintenance dose needs to be much smaller (100 to 200 mg) than when lamotrigine is used either alone or with enzyme-inducing AEDs, such as carbamazepine.
j. Use of lamotrigine, topiramate, or zonisamide with cytochrome oxidase enzyme inducers such as phenytoin or carbamazepine would increase the clearance of the formal group of AEDs needing higher doses compared to when used as monotherapy.
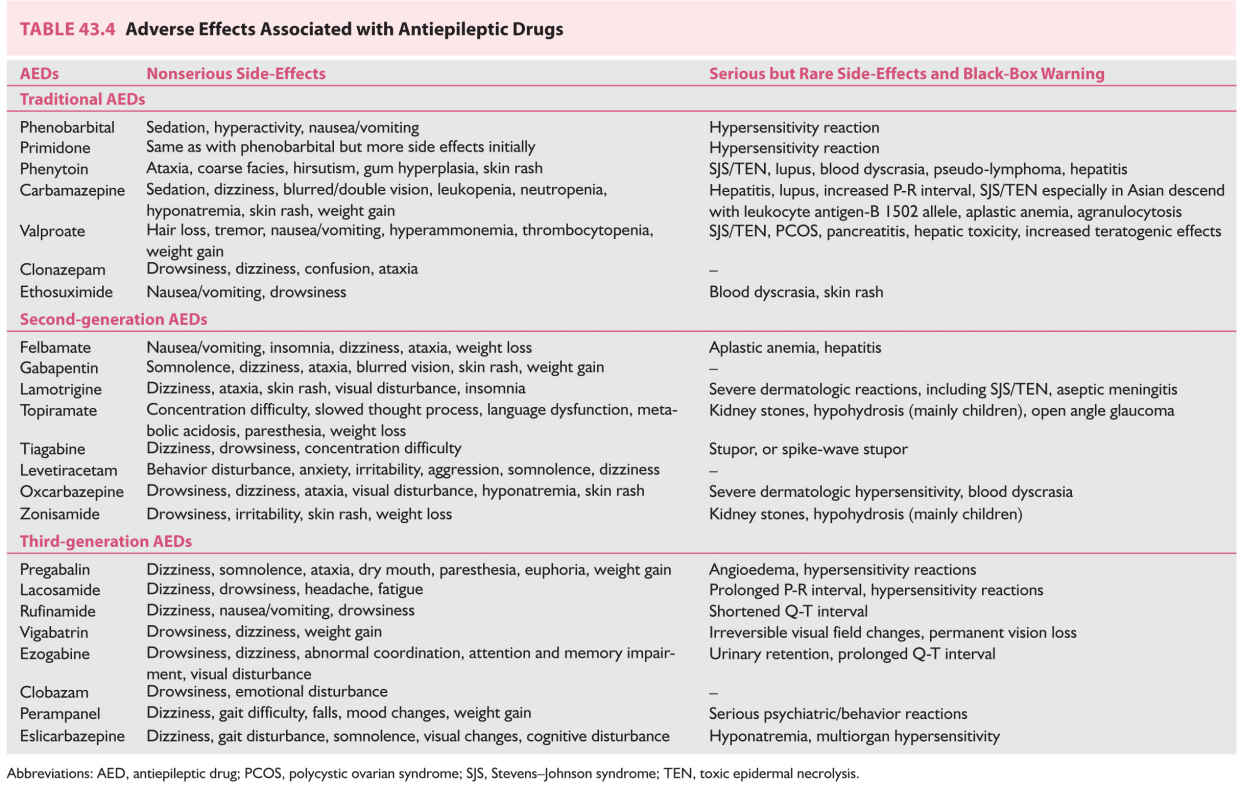
D. Side effects of AEDs. These are summarized in Table 43.4.
1. Traditional AEDs.
a. All traditional AEDs are associated with neurotoxic side effects such as tiredness, somnolence, dizziness, ataxia, blurred or double vision, nystagmus, and difficulty with concentration, behavioral disturbances, and cognitive dysfunction.
b. Phenobarbital, primidone, and clonazepam have higher incidence of sedative/hypnotic side effects.
c. Phenytoin has several cosmetic side effects such as facial coarsening, facial/body hirsutism in women, and gum hyperplasia. These are concerning particularly to women.
d. Valproic acid, when used in larger doses, is associated with tremor.
e. Valproic acid and carbamazepine are commonly associated with weight gain.
f. Serious reactions to traditional AEDs are reported but are uncommon. These include hepatotoxicity, bone marrow suppression (aplastic anemia and agranulocytosis), exfoliative dermatitis, and Stevens–Johnson syndrome. HLA-B*1502 is associated with the risk of rash from carbamazepine. Valproic acid is also reported to produce pancreatitis. If not detected in time, these reactions may be fatal. Hence, periodic monitoring of hepatic and hematopoietic parameters is recommended, although there is no evidence that routine testing detects these serious conditions much prior to a time when the effects can still be prevented. Usually benign leukopenia occurs in 10% to 20% patients, especially on carbamazepine. A total white cell count of 2,000 per mm3 and an absolute granulocyte count of 1,000 per mm3 are well tolerated.
Valproic acid is reported to produce thrombocytopenia, interference in the platelet aggregation, and an increased tendency toward bleeding. However, platelet counts above 60,000 per mm3 are acceptable.
g. Because of its inhibition of cytochrome P-450 system, valproic acid in women is associated with abnormal metabolism of gonadal and adrenal sex hormones, often resulting in PCOS. PCOS is characterized by infertility, hirsutism, obesity, increased serum androgen levels, frequent anovulatory cycles, and other menstrual abnormalities. The incidence of PCOS is 10% to 20% in women with epilepsy compared with 5% to 6% of women in the general population. The incidence of this syndrome is highest in women with PGE treated with valproic acid.
h. Teratogenic effects occur in 5% to 10% pregnancies with the use of many traditional AEDs, highest incidence with valproic acid.
i. Almost all of the traditional AEDs are associated with bone loss, even in young adults, and affect both genders following their use of 1 to 2 years.
2. Newer AEDs.
a. Many of the newer AEDs (e.g., oxcarbazepine, gabapentin, zonisamide, and tiagabine) are associated with neurotoxic side effects similar to those with traditional AEDs. Lamotrigine, levetiracetam, topiramate, and lacosamide have relatively less neurotoxicity in the commonly used daily doses.
b. Serious reactions are rare with newer AEDs with the exception of a high incidence of felbamate-associated aplastic anemia and toxic hepatitis detected after its release. This has led to a marked drop in its use and presently it is used only in medically refractory epilepsy, which has failed to respond to conventional AEDs.
c. Lamotrigine causes a skin rash in 5% to 10% of patients, and even Stevens–Johnson syndrome may occur, although rarely. These complications are more common with rapid titration of the dose and comedication with valproic acid.
d. Topiramate produces cognitive dysfunction, for example, slower mentation, and word-finding difficulty, problems in concentration, thinking abnormalities, impaired memory, and encephalopathy. These are less common with daily doses below 400 mg. Rarely, acute myopia associated with the secondary angle closure glaucoma has been reported.
e. Levetiracetam is associated with anxiety and behavioral changes. Similarly, parampanel is reported to produce severe psychiatric and behavioral reaction in significant proportion of patients.
f. Zonisamide and topiramate may produce renal calculi in approximately 1% patients, paresthesiae in the limbs and hypohyderosis.
g. Like carbamazepine, oxcarbazepine and eslicarbazepine are associated with significant hyponatremia in 1% to 3% of patients, more often in elderly patients or those who are on other medications. It becomes evident in the first few months of therapy but rarely necessitates discontinuation of the drug.
h. Lacosamide may cause prolonged P–R interval; hence, it should be used with caution in patients with known cardiac conditions.
i. Like several other drugs, lamotrigine is found to be associated with aseptic meningitis, which can manifest with headaches, meningismus, and low-grade fever.
j. Multiple AEDs are more likely to cause cognitive side effects such as sedation, dizziness, diplopia, and blurred vision. Common examples of such combinations include the use of carbamazepine or oxcarbazepine with lamotrigine or lacosamide. Reducing the dose of any one of the combination AED may eliminate or reduce the side effects.
k. There has been little available data regarding the effect of newer AEDs on bone density or teratogenicity. Most recent data of the North American Pregnancy Registry strongly suggests that lamotrigine, levetiracetam, and oxcarbazepine when used as monotherapy have lower teratogenic potential than that with many traditional AEDs (valproate, phenobarbital, phenytoin, and carbamazepine).
3. Extended-release formulations. It is well known that adherence to an AED increases from less than 50% for four-daily dosing to more than 80% with one-daily dosing. While adherence is best with one-daily dose, the risk of a breakthrough seizure may also be more after missing a dose. Extended-release products slow the absorption of drugs that showed elimination half-life, allowing only one or two daily dosing and more stable blood levels and more adherence. These formulations are now available for many AEDs including phenytoin, carbamazepine, valproate, oxcarbazepine, topiramate, lamotrigine, and levetiracetam. Extended-release products of carbamazepine and valproate are perhaps most useful because these AEDs given in a single or two daily doses have high tolerability than with multiple daily dosing of the parent drugs essentially in elderly population.
FIRST SEIZURE
AED therapy is usually not initiated after the first tonic–clonic seizure and postponed until a second seizure occurs when the diagnosis of recurrent seizures or epilepsy is made. The incidence of recurrence is 25% to 65% after the first and over 75% after the second seizure. Hence, the first seizure may be an isolated episode and not necessarily herald the onset of epilepsy. This is particularly true if a single tonic–clonic seizure was related to sleep deprivation, physical or mental stress, drug or alcohol withdrawal, or use of prescribed (e.g., Wellbutrin) or recreational drugs (e.g., cocaine). Overall, 50% of patients have recurrence over a 3-year follow-up period after the first tonic–clonic seizure. The incidence is <25% among subjects with low-risk factors to 65% or more for those with two or more of the following risk factors: strong family history of seizures in siblings, history of febrile convulsions, focal-onset seizure, postictal paralysis, abnormal cognitive and neurologic examinations, evidence of a structural cerebral lesion at neuroimaging, and the presence of epileptiform abnormalities at EEG. Patients with two or more of these risk factors therefore may need prompt initiation of AED therapy even after the first tonic–clonic seizure. American Academy of Neurology (AAN) practice parameter (2015) recommends EEG and imaging studies (MRI preferred) in the evaluation of adults presenting with the first unprovoked epileptic seizure. Management of newly diagnosed epilepsy by AED regime requires special consideration. The choice is certainly based on seizure type, epilepsy syndrome, comorbidity, concomitant medications, possible interactions, safety during pregnancy, and side-effect profile. A proper choice may improve compliance, minimizing the time before resuming driving and baseline activities. Also, once an AED is started, the clinician may be reluctant to change the AED and more likely to add another AED if the patient responds poorly, risking more side effects. Levetiracetam is a new-generation AED, whose use has increased tremendously owing to its ease of use, linear pharmacokinetics, lack of interaction with other drugs and broad spectrum of efficacy. In addition, there is accumulating evidence of the safety of levetiracetam in pregnancy. Several studies comparing levetiracetam with other AEDs have confirmed that it has similar but not any better efficacy compared to carbamazepine, valproate, oxcarbazepine, and others as the first-line therapy in a patient with newly diagnosed epilepsy.
STATUS EPILEPTICUS
A. Definitions. Status epilepticus (SE) is usually considered to be present if continuous seizure activity persists for at least 30 minutes or two or more sequential seizures repeat within 30 minutes without full recovery of consciousness between seizures. Because the neuronal injury increases with duration, experts in recent literature define SE if seizure activity persists beyond 5 minutes, or, if at least two seizures have occurred over this period without full recovery. The term established SE is applied to when it remains unresponsive to first line of medication (benzodiazepines), refractory SE refers to the one which fails to get under control by “first” and “second” line of medications (benzodiazepines and AEDs), and the term super-refractory SE is often applied to that which persists beyond 24 hours after the start of “third” line of drugs (general anesthesia).
B. Types. Overall incidence is 2 to 4 per 10,000 per year. Any type of seizure can manifest as SE, but the common forms include the following:
1. Generalized convulsive status epilepticus (GCSE) manifests as repeated major motor convulsions without full recovery of consciousness between seizures. In the past, the term SE implied essentially to this form of status.
2. Nonconvulsive SE produces a continuous or fluctuating “epileptic twilight” state. This includes absence status and complex partial status. Only an EEG can establish the diagnosis.
3. Simple partial SE is characterized by repeated focal motor seizures, epilepsia partialis continua, and focal impairment of function (e.g., aphasia) without accompanying alteration of consciousness.
C. Cause of GCSE. GCSE is the most common and most serious type of SE. It occurs mainly in the following settings:
1. Acute cerebral insult or acute encephalopathy accounts for one-half of cases of GCSE. These disorders include meningitis, encephalitis, head trauma, hypoxia, hypoglycemia, drug intoxication (e.g., cocaine), drug withdrawal, and strokes or metabolic encephalopathy.
2. GCSE can occur in patients with a history of chronic epilepsy, the common precipitants being changes in AEDs, sudden discontinuation or reduction in AEDs, systemic infection, physical and emotional stress, and sleep deprivation.
3. It can occur as an initial unprovoked epileptic event in an otherwise healthy person. Such “idiopathic” cases may account for one-third of all cases of GCSE.
D. Prognosis. GCSE is an emergency associated with substantial morbidity and mortality.
The overall mortality may be as high as 30% among adults. GCSE associated with acute neurologic insults has the poorest prognosis, which essentially depends on the underlying cerebral etiologic factor. When GCSE is the first epileptic event for an otherwise neurologically intact patient, or when it occurs in a patient with chronic epilepsy, or because of drug or alcohol withdrawal, the prognosis is good if therapy is instituted promptly. Without adequate and prompt treatment, GCSE can progress to a state of electromechanical dissociation in which the patient becomes increasingly unconscious or encephalopathic from the ongoing status, but the convulsive activity becomes increasingly subtle although EEG continues to show an ictal pattern. Patients with this condition, which is often called subtle SE, are considered to be candidates for an aggressive therapy, as are those with overt GCSE.
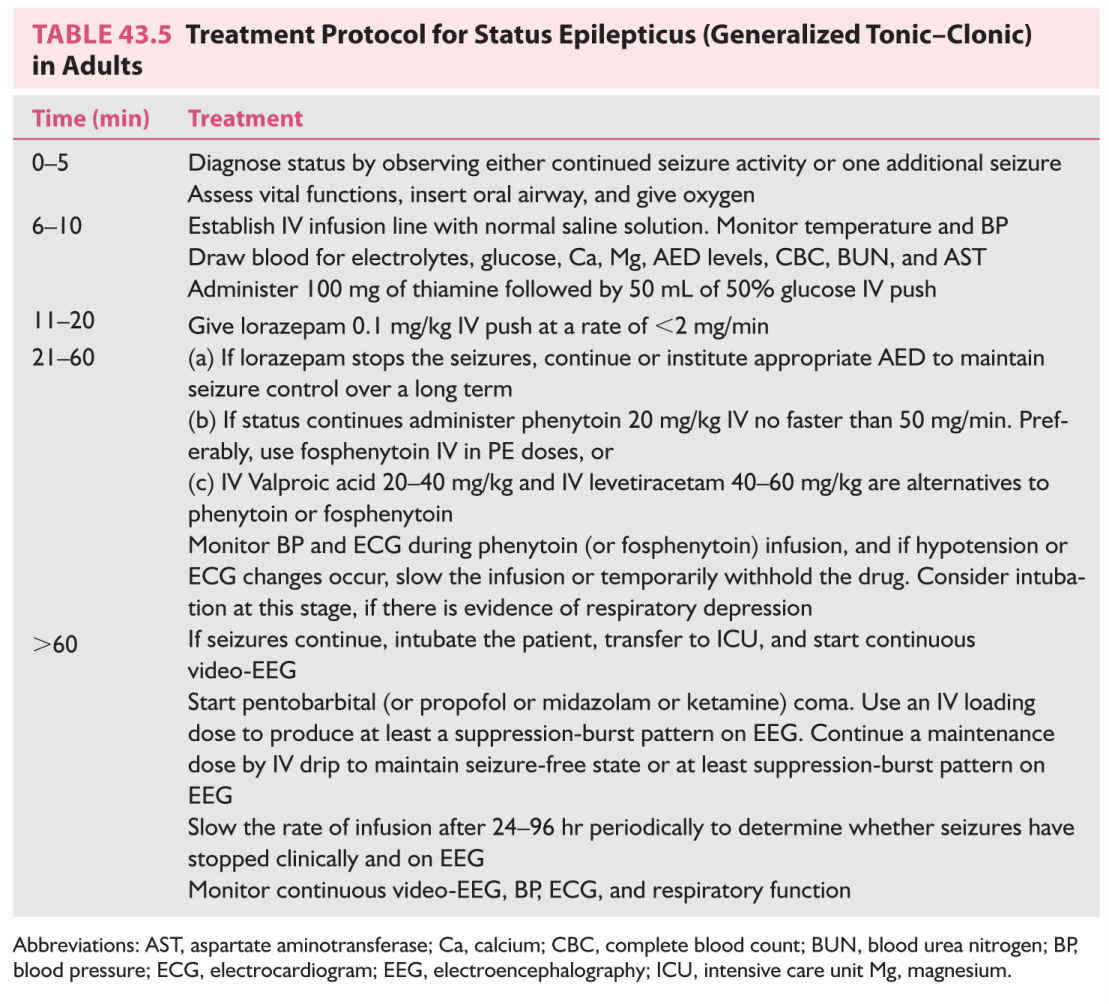
E. Management of GCSE. The treatment protocol outlined in Table 43.5 is useful in the management of GCSE.
1. Diagnose SE by observing either continued seizure activity beyond 5 minutes or two generalized convulsions without full recovery of consciousness.
2. Assess vital functions and systemic abnormalities and stabilize the vital functions as much as possible.
a. Maintain an adequate airway and oxygenation. This can usually be accomplished with an oral airway. The airway should be suctioned periodically to maintain patency. Oxygen should be administered through a nasal cannula or with a mask and a bag-valve-mask ventilator. If, after bagging, respiratory assistance is still needed, endotracheal intubation should be considered.
b. Assess blood pressure (BP) and maintain it at a normal or high-normal level during prolonged GCSE. Use vasopressors if necessary.
c. Establish an IV infusion line using normal saline solution. Blood should be drawn initially for CBC, blood sugar, blood urea nitrogen (BUN), serum electrolytes (including calcium and magnesium), and AED levels, and both urine and blood should be obtained for toxicology screening.
d. Assess oxygenation by means of oximetry or periodic arterial blood gas determination.
e. Monitor rectal temperature. Body temperature can increase to a high level during prolonged SE as a result of increased motor activity.
f. If hypoglycemia is documented or if it is impossible to obtain prompt blood sugar determination, administer 50 mL of 50% glucose by means of IV push. In adults, thiamine (100 mg) is always given before glucose to protect a thiamine-deficient patient from exacerbation of Wernicke’s encephalopathy.
g. Administer bicarbonate therapy only if serum pH is so low as to be immediately life-threatening. Acidosis commonly develops during GCSE, but acidosis usually responds promptly once the seizure activity is controlled.
h. For rare patients with GCSE resulting from hyponatremia (serum sodium concentration <120 mEq/L), hypocalcemia, or hypomagnesemia, administer appropriate electrolytes by means of IV drip.
3. Drug therapy for the control of GCSE. The goals of therapy are rapid termination of the clinical and EEG evidence of seizure activity and subsequent maintenance of a seizure-free state. At present, the drug treatment of GCSE is subdivided into three lines: the first line of treatment consisting of short-acting benzodiazepines, the second line comprising various AEDs, and the third line consisting of general anesthesia. The use of IV lorazepam (2 mg), diazepam (5 mg), or midazolam by the emergency medical personnel prior to arrival in the emergency room is increasingly recommended for out-of-the-hospital status. In the emergency room, most physicians in the United States prefer IV lorazepam as the first line of treatment.
a. Lorazepam is administered at 0.1 mg/kg IV at a rate of 1 to 2 mg/minute. Lorazepam has relatively rapid effectiveness and yet has a prolonged duration of action against SE; hence, it has replaced diazepam almost completely for the treatment of SE. Lorazepam like diazepam can produce serious respiratory depression or hypotension, particularly when given in combination with barbiturates.
GCSE responds to first-line treatment in approximately two-thirds of the patients. They will need to be treated further with appropriate AEDs to maintain seizure control. In the benzodiazepine refractory SE (established SE), one of the many IV antiepileptic medications can be used as the second line of treatment, including phenytoin, fosphenytoin, valproate, phenobarbital, levetiracetam, and lacosamide. A multicenter, randomized trial comparing the efficacy of fosphenytoin, valproate, and levetiracetam has been recently initiated but the preliminary studies suggest that these three AEDs may be equally effective. At present, fosphenytoin remains the most commonly used second-line treatment.
b. Phenytoin or fosphenytoin. The usual loading dose of phenytoin is 20 mg/kg, given through a syringe into the IV port close to the patient at a rate <50 mg/minute. Injection is preferably performed by a physician. BP and electrocardiogram (ECG) are continuously monitored throughout the infusion. The rate of infusion should be slowed or temporarily stopped if hypotension, widening of the QT interval, or arrhythmias develops.
There is a significant risk of skin complications when IV phenytoin is given. These include phlebitis, tissue sloughing after extravasation, and most serious, purple glove syndrome after infusion into a dorsal hand vein. The latter is a delayed soft tissue injury that can result in severe edema, arterial occlusion, and tissue necrosis that can necessitate amputation. These complications occur because parenteral phenytoin has 40% propylene glycol (antifreeze). Its pH is adjusted to 12.2 with sodium hydroxide (drain cleaner). Use of IV phenytoin is now virtually replaced by fosphenytoin, a much safer preparation for IV administration.
Fosphenytoin, a phosphate ester of phenytoin, is enzymatically converted to phenytoin by serum phosphatases. It is available only for parenteral use (IV or IM). Because it is dissolved in TRIS buffer at a pH of 8 to 9, fosphenytoin does not cause tissue injury as phenytoin does. Three parts of phenytoin are bioequivalent to two parts of phenytoin, but the fosphenytoin dose is labeled in phenytoin equivalents (PE), so that 150 mg of fosphenytoin is labeled 100 mg PE. Although somewhat confusing, it apparently allows easy conversion of phenytoin to fosphenytoin dosing. Fosphenytoin can be administered more rapidly, up to 150 mg PE/minute compared with <50 mg/minute for phenytoin. The shorter infusion time compensates for the time needed for its conversion to active phenytoin. The result is that peak levels of phenytoin are attainable with IV fosphenytoin as rapidly as with phenytoin infusion itself. BP and ECG monitoring are recommended during fosphenytoin infusion, as they are with IV phenytoin. Valproate is given intravenously in doses of 20 to 40 mg/kg over 10 minutes, whereas levetiracetam in doses of 40 to 60 mg/kg over 5 to 10 minutes. Because of its relative ease of administration and no required monitoring, levetiracetam may be an attractive alternative to fosphenytoin.
c. General anesthesia. GCSE is successfully terminated in approximately 75% of patients by lorazepam and phenytoin (or fosphenytoin) combination. Remaining 25% constitute refractory GCSE. There is a high probability that the patient has an acute cerebral insult responsible for SE and prognosis is more guarded, with mortality being around 50%. The third line of treatment is general anesthesia.
(1) The patient must be intubated (if not already) and placed in intensive care unit (ICU) for managing refractory GCSE.
(2) Continuous video-EEG monitoring must be initiated to detect subtle or electrographic status and to evaluate the effectiveness of general anesthesia.
(3) The goal of general anesthesia is to eliminate not only clinical seizures but also electrical discharges indicative of continuing seizure activity. Continuous IV infusion of pentobarbital, propofol, or midazolam has been reported to be almost equally effective. The choice of a drug is largely based on the clinical experience of the physician. If one agent in optimal dosages is unsuccessful in controlling status, another should be tried. The rate of administration of the drug is adjusted to ensure cessation of all epileptiform activities, or at least suppression-burst pattern in the EEG. However, maximal EEG suppression is associated with an increased frequency of hypotension requiring the use of pressor agents. The anesthesia is continued for 1 to 2 days before an attempt is made to lighten it. If clinical seizures or ictal EEG patterns return, the infusion is appropriately increased.
(4) Anesthetic agents. Pentobarbital has been used for a long period for refractory GCSE. It is started intravenously with a loading dose of 5 to 15 mg/kg, followed by a maintenance dosage of 0.5 to 1.0 mg/kg/hour.
Propofol, a short-acting agent, is becoming the drug of choice. It is started IV with a loading dose of 1 to 2 mg/kg, given over 5 minutes, followed by a maintenance dosage of 1 to 5 mg/kg/hour.
Midazolam is administered at a loading dose of 0.2 mg/kg by slow IV bolus followed by a maintenance dose of 0.1 to 0.5 mg/kg/hour.
There are no prospective randomized trials comparing these three anesthetic agents for the treatment of refractory status. One systematic review demonstrated no difference between the three, at least for mortality rate. Ketamine may be another anesthetic agent effective in refractory SE; hypotension and cardiopulmonary depression may be less common, so that larger doses may be used to control status.
4. Long-term antiepileptic therapy. After the episode of GCSE has been brought under control, most patients need continuation of some form of AED therapy. Long-term AED therapy is indicated when GCSE is caused by a structural brain lesion or when the patient has a history of epileptic seizures. When GCSE constitutes the patient’s first seizure and no cause is found, the decision to initiate long-term AED therapy should be individualized, but most physicians initiate long-term treatment under such circumstances. If the GCSE was caused by acute CNS involvement, such as metabolic encephalopathy, meningoencephalitis, or cerebrovascular compromise, antiepileptic therapy is continued for a short period of 3 to 6 months.
F. Management of other types of SE. Other forms of SE do not pose the same emergency situation that GCSE poses. Complex partial SE has been reported to result in long-term neurologic deficits (e.g., permanent memory impairment) and should be controlled promptly with a benzodiazepine (e.g., lorazepam) followed by IV phenytoin (or fosphenytoin) or IV levitaracetam. IV anesthesia is rarely indicated. Absence status is best managed with an IV benzodiazepine (diazepam or lorazepam), which is effective in most cases. If a benzodiazepine is not effective, valproic acid can be given intravenously, in a dose of 25 mg/kg to promptly achieve therapeutic blood levels. After the absence status is controlled, valproic acid therapy is continued orally. Simple partial status, focal motor status or epilepsia partialis continua without loss of consciousness responds to phenytoin (or fosphenytoin) usually in large doses to maintain blood levels as high as 30 mg/mL. IV levitracetam, valproic acid, and lacosamide are other alternatives. Benzodiazepines are not desirable because of their sedative side effects, except using one or two doses of IV lorazepam or diazepam initially.
MANAGEMENT OF ACUTE SEIZURE CLUSTER
Some patients have repetitive series or clusters of epileptic seizures that occur within a short period, not meeting the definition criteria of SE. Such seizure clusters can be intermittently managed with either of the following approaches:
A. Most physicians use benzodiazepine (lorazepam or diazepam) to manage a cluster. Lorazepam is given in doses of 2 to 4 mg orally (sublingually) or parenterally (IM or IV), usually becoming effective in approximately 30 minutes. Administration may be repeated in doses of 1 to 2 mg with a maximum dose of 6 to 8 mg in 24 hours.
B. Rectal diazepam (Diastat) is approved for rectal administration in the management of repetitive seizures. It is as effective, if not better, than lorazepam. Diazepam gel is administered in doses of 0.2 to 0.5 mg/kg rectally (usual adult dose is 10 to 20 mg); it is effective in 15 minutes and the effect lasts as long as 8 hours. If social or logistic reasons make the use of rectal administration difficult, sublingual, intranasal, or oral lorazepam is a better alternative.
C. Intranasal diazepam (0.2 mg/kg) may be more easy to deliver and acceptable than rectal diazepam. Caregiver can give the drug more easily and administer during or immediately after a seizure. Preliminary studies have shown that the intranasally delivered drug appears to achieve a peak concentration high enough to be therapeutically effective.
Home treatment with diazepam reduces the risk for subsequent status and the need for frequent visits to the emergency room in patients at risk for seizure clusters or SE.
EPILEPSY, PREGNANCY, AND OTHER WOMEN’S ISSUES
A. Major problems. Pregnancy in a woman with epilepsy (WWE) is considered to constitute a high risk and needs to be followed by a high-risk obstetrician. Recently, AAN has issued guidelines regarding the management of WWE, updated in 2009, which are incorporated in the following discussion:
1. Obstetrical complications. In short, there is no conclusive evidence of increased obstetrical complications in WWE on AEDs.
a. There is a weak evidence that WWE on AEDs has a slightly higher (up to 1.5 times expected) risk for cesarean section but a good evidence that this risk is not >2 times expected.
b. There is a good evidence that there is no greater risk for late pregnancy bleeding, early contractions, or early labor and delivery in WWE on AEDs. However, these complications are substantially increased in WWE who smoke.
c. There is insufficient evidence to support or refute an increased risk for preeclampsia, hypertension, or miscarriage.
2. Effect of pregnancy on epilepsy. It is usually believed that seizures become more frequent during pregnancy in one-third WWE, especially in second and third trimesters. According to the AAN guidelines, there is insufficient evidence to support or refute an increased risk in seizure frequency or occurrence of SE. Furthermore, if a WWE has been seizure free for at least 9 months before becoming pregnant, there is a high likelihood of her remaining seizure free during pregnancy. Nonadherence to AEDs is common during pregnancy because of inadequate knowledge of the AED-induced teratogenesis. Also, the ill effects of tonic–clonic seizures do occur on the fetus and these points need to be fully discussed.
3. Alteration of the pharmacokinetics of AEDs (increased clearance) results in decreased serum concentrations of almost all AEDs. AAN guidelines (2009) concluded that there is a probable chance of decrease in the serum concentration of lamotrigine, phenytoin, and to a lesser degree carbamazepine, whereas a possible chance for decrease of levetiracetam and the monohydroxy derivative of oxcarbazepine. Since lamotrigine is metabolized by glucuronidation (most activated during pregnancy), its clearance is most enhanced needing an upward dosing. The clearance normalizes during the last week of pregnancy necessitating dose reduction immediately after delivery to avoid drug toxicity. Serum concentration of most other AEDs increased gradually over 4 to 8 weeks postdelivery. Changes in AED levels and in the hormonal status, poor compliance, psychological stresses, and sleep deprivation during pregnancy are some of the factors for exacerbation of seizures in some WWE.
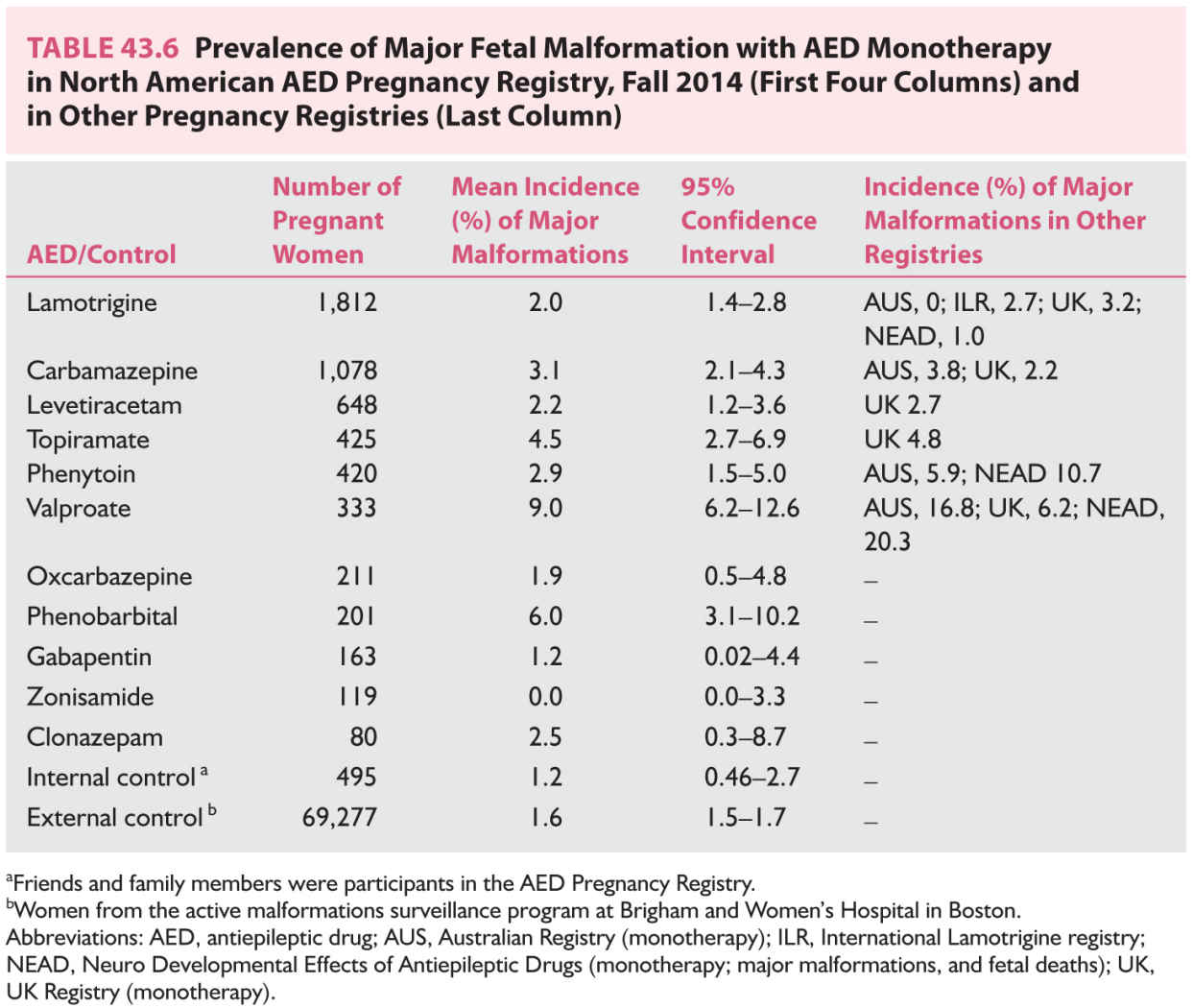
4. The incidence of both minor and major fetal malformations is two to three times higher among WWE than among women without epilepsy. Minor malformations are deviations from normal morphology not requiring treatment, whereas major malformations, which are most relevant, require medical and/or surgical treatment. The overall incidence of major malformations (oropalatal clefts, urogenital and congenital heart anomalies, and neural tube defects) among infants of epileptic mothers is approximately 3% to 10%, compared with 1.5% to 3% in women not receiving AEDs. Most of these teratogenic effects are due to intrauterine exposure to AEDs, especially during the first trimester when neural tube (4 weeks), cardiac (6 weeks), and orofacial (6 to 10 weeks) developments are happening. All major AEDs have potential teratogenic effects, but the incidence varies. The most recent prevalence data from the North American Pregnancy registry are shown in Table 43.6. There are other pregnancy registries providing similar conclusions. The findings are summarized below and are in line with the AAN practice parameter (2009):
a. Incidence of malformation increases with polytherapy compared with monotherapy. Avoid polytherapy during pregnancy.
b. Valproic acid is associated with the highest incidence (10%) of major malformations, which include neural tube defects, facial clefts, and hypospadias. Hence, avoid the use of valproic acid in women during childbearing years.
c. In general, increased dose of AED correlates with higher incidence of major malformation, this being particularly the case with valproic acid, but also for carbamazepine, lamotrigine, and phenobarbital. Therefore, use lowest effective dose of AED during pregnancy.
d. Phenytoin, carbamazepine, and phenobarbital also increase the risk of fetal malformation: cleft palate with phenytoin use, posterior cleft palate with carbamezapine use, and cardiac malformation with phenobarbital use. Hence, avoiding these AEDs during pregnancy would reduce major malformations.
e. Lamotrigine, which is the most extensively studied new AED, appears to have the least teratogenicity (2% to 3%), probably no different from the general population. The UK registry has also shown dose–response effect of lamotrigine-associated malformations, 2% incidence with doses up to 200 mg/day compared with 5.4% for above 200 mg/day. Polytherapy use of lamotrigine with valproic acid markedly increases the incidence of malformation (12%).
f. UK and North American registries have shown higher incidence (4% to 5%) of major malformations (oral clefts, hypospadias) with topiramate, whereas a lower incidence with levetiracetam and zonisamide. Topiramate should, therefore, be avoided during pregnancy.
5. Adverse perinatal outcomes. There is good evidence that WWE taking AEDs have an increased risk of small-for-gestation offspring, a weak evidence for low Apgar scores at 1 minute, and a good evidence of no increase in the risk of perinatal death. Hemorrhagic disease is reported in newborns delivered by women who have received hepatic enzyme-inducing AEDs (phenobarbital, primidone, phenytoin, and carbamazepine) because of their effect in decreasing the vitamin K-dependent clotting factors. According to the AAN guidelines, there is insufficient evidence to support or refute an increased risk of hemorrhagic complications in the newborn of WWE taking AEDs.
6. Cognitive outcomes. An AAN practice parameter update (2009) concluded that children born to women, who received valproate (good evidence), phenytoin, or phenobarbital (weak evidence), have higher incidence of poor cognitive outcome compared with the offspring of WWE not taking AEDs. Carbamazepine does not have adverse effect. AED polytherapy is more likely associated with cognitive impairment compared to monotherapy. A recent study comparing monotherapy with carbamazepine, valproate, phenytoin, or lamotrigine demonstrated increased developmental delays and lower IQ at age 6 years in valproate-exposed children as well as reduced verbal IQ and increased need for special education in these children at age 6 years or above. The adverse cognitive development appears dose-dependent. The cognitive teratogenesis associated with valproate is another reason to avoid valproate in women who want to or may become pregnant.
B. Management guidelines. The therapeutic challenge is to keep the patient free of seizures while minimizing the adverse effects of seizures and AEDs on the course of pregnancy and the fetus. The major guidelines, which are preferably initiated before the patient becomes pregnant, are as follows:
1. Counsel the family about the higher incidence of fetal malformation, but assure the patient that >90% exposed to AEDs still bear healthy offspring.
2. May consider withdrawing AEDs before pregnancy if the patient has remained free of seizures for >2 years unless the woman has a high probability of recurrence.
3. Before conception, determine the best AED for seizure control. If the patient is on polytherapy, reduce AEDs to appropriate monotherapy. Because of the relatively high incidence of neural tube defects with valproic acid, avoid these AEDs and replace with other AEDs. For women with PGE, lamotrigine alone or with levetirecetam or zonisamide are alternative AEDs.
4. Prescribe folic acid in any sexually active woman during childbearing age to reduce the overall incidence of neural defects in the offspring.
5. Address and eliminate other risk factors, for example, drugs, alcohol, and smoking.
6. Do not stop or change AED therapy after the pregnancy has been diagnosed. The risk of fetal malformation is highest during the first 4 to 8 weeks of pregnancy. It is usually too late to protect the fetus by the time pregnancy is confirmed. Stopping or changing the drug can induce more frequent and more violent seizures with adverse consequences on both the mother and the fetus.
7. Supplemental multivitamins and folic acid are prescribed during the entire pregnancy. The precise dose for WWE has not been defined, but 1.0 mg/day is commonly used. Women at risk of having a child with neural tube defect (previous child or family history) are advised to supplement with 4.0 mg/day.
8. Monitor the AED serum level (preferably free level) before conception, at each trimester, in the last month of pregnancy, and through 8 weeks postpartum, especially if the patient is on lamotrigine, carbamazepine, or phenytoin. Adjust the dosage accordingly. Maintain lowest effective blood levels.
9. Measure serum α-fetoprotein and acetylcholinesterase levels at 15 to 18 weeks of gestation to be followed by high-definition ultrasound imaging to detect neural tube defects and other major malfunctions. Together, these detect over 99% of the major fetal abnormalities. Amniocentesis is indicated only if these tests do not provide positive exclusion of a neural tube defect.
10. According to AAN practice guidelines (2009), there is insufficient evidence to support or refute a benefit of vitamin K supplement for reducing the risk of hemorrhagic disease of the newborn of WWE. To the newborns exposed to enzyme-inducing AEDS in utero, administer vitamin K1 (1.0 mg IM) to the neonate immediately after birth as is the routine practice for all neonates.
11. After delivery, check AED levels and adjust the doses of AED, usually at a lower level. Some AEDs, such as lamotrigine and oxcarbazepine, start reversing their elimination rate to the prepregnancy level even prior to the delivery. Their dose needs to be brought down within a week or so of the delivery.
12. Breastfeeding is allowed. Nearly all AEDs appear in breast milk at a concentration closely related to the AED’s free plasma level. Hence, highly protein-bound AEDs attain only a very small concentration in the milk to produce significant clinical effects on the breast-fed baby. AAN practice parameters (2009) conclude that valproate, phenobarbital, phenytoin, and carbamazepine do not transfer into breast milk to as great an extent as gabapentin, lamotrigine, and topiramate. Levetiracetam and ethosuximide also appear in sufficient concentration in breast milk. The AAN practice parameter states that the clinical consequences for the newborns exposed to AEDs in the breast milk remains unknown. A recent Norwegian study found long breastfeeding as safe to infants exposed to carbamazepine, lamotrigine, or valproate in the breast milk. Data are still lacking for newer AEDs, which are now increasingly used such as levetiracetam, oxcarbazepine, topiramate, and zonisamide. It may take several years and extensive studies to establish the safety of these recently introduced AEDs regarding breastfeeding.
C. Prevention of pregnancy with oral contraceptives.
1. Women with epilepsy may use oral hormonal contraceptives if they wish. These do not exacerbate epilepsy despite the warnings on package inserts.
2. The major concern in using oral contraceptives is the higher failure rate (>6% per year) among women taking hepatic cytochrome-450 isoenzyme-inducing AEDs (Table 43.3 last column). This is due to increased elimination of both estrogens and progestogens by these AEDs. The effect is on many contraceptive preparations including combined contraceptive pill, combined patch, progestogen-only pill, and progestogen implants.
The most commonly used is a combined “mini-pill” containing 35 mg or less of estrogen combined with progestogen. It may be less effective. Breakthrough bleeding can be a warning of decreased contraceptive efficiency. AAN guidelines recommend that patients taking these enzyme-inducing AEDs be placed on a medium-dose combined contraceptive pill containing at least 50 mg of ethinyl estradiol. Additional use of condom is strongly recommended to assure maximum contraception efficacy. The progestogen-only pill is usually ineffective.
Long-acting reversible contraceptives are other desirable alternatives in patients on enzyme-inducing AEDs. Progestogen-only implants are not effective. Intramuscular medroxyprogesterone injections (Depo-Provera) appear to be very effective (>99%) but patients are advised to take injections every 10 weeks rather than 12 weeks if they are on enzyme-inducing AEDs. However, it is associated with side effects of weight gain, decreased bone mineral density (BMD), and delayed return of fertility. Contraception using levonorgestrel-releasing intrauterine device (Mirena coils) is effective for a period up to 5 years and is not affected by enzyme-inducing AEDs because progestogen acts by being released locally in the uterus. It is a highly effective (>99%) contraceptive option in women with poor compliance history.
Because both estrogens and progestogens are eliminated faster by enzyme-inducing AEDs, the higher dose of estrogen needs to accompany a similarly higher dose of progestogen in the combined contraceptive pill.
3. Nonenzyme inducers are unlikely to cause failure of oral contraception. Refer to Table 43.3.
4. Hormonal contraceptives have little effect on AEDs except on lamotrigine blood levels, which drop to half the precontraceptive levels because of enzyme induction. During the 7-day-off period, the levels could double back to prehormone levels. This effect may result in a breakthrough seizure during the 3 weeks of hormonal ingestion and toxicity during the off-week.
D. Catamenial epilepsy. Many women have increased frequency of seizures related to their menstrual cycle probably because of changing hormonal concentration during menstrual cycle. The term catamenial epilepsy is usually applied if seizure frequency increases by two times or more in relation to specific times of menstrual cycle. It happens in about one-third of women with partial epilepsy and increased seizures occur at three different times. Commonly, they occur either just before or during the first few days of menstruation (perimenstrual). Some have increased seizures at ovulation (midcycle), whereas others who have anovulation cycles have increased seizure activity in the second half of the menstrual cycle. Acetazolamide (250 to 500 mg/day) or clobazam (5 to 15 mg/day) may be used during the periods of increased seizure activity. An alternative is to give additional doses of the AED or use a benzodiazepine such as lorazepam for a few days. Hormone therapy is another alternative approach. Medroxyprogesterone acetate can be effective, but has undesirable side effects of weight gain and bone density loss. Progestogen supplements may be given perimenstrually or during the latter half (10 to 26 days) of the menstrual cycle. A combined oral contraceptive pill or depot progestogen may also be used.
BONE MINERAL DENSITY
Almost all of the traditional AEDs are associated with bone mineral density (BMD) loss, even in young adults, and affect both genders following their use of more than 1 year. The bone loss is more severe in elderly patients, with polytherapy and with prolonged use. Possible mechanisms include hepatic induction of cytochrome P-450 enzymes (phenobarbital, primidone, phenytoin, and carbamazepine) leading to increased catabolism of vitamin D, secondary hyperparathyroidism, and increased bone turnover. Valproic acid, a noninducer, does produce bone loss by increased ostoclastic activity. There is no reliable data regarding BMD loss with newer AEDs, and oxcarbazepine may not be better in this respect compared with carbamazepine.
Reduced BMD and epilepsy increase the risk, two to six times, for fractures, particularly of the hip and spine. Bone loss is best detected by dual-energy X-ray absorptiometry (DXA) and expressed as a T-score. Osteopenia is defined by a T-score of −1 to −2.5 and osteoporosis by a score −2.5 or over.
All adults with epilepsy, especially on enzyme-inducing AEDs, should receive 1,000 to 1,500 mg/day of calcium in addition to 800 to 1,600 IU of vitamin D. DXA should be performed, probably every 4 to 5 years to assess BMD loss especially in epileptic patients at greater risk (elderly, postmenopausal women on enzyme-inducing AEDs). If found to have low BMD, treatment with bisphosphonates (alendronate and risedronate), raloxifene, teriparatide, or hormonal replacement in postmenopausal women may be considered.
ANTIEPILEPTIC DRUG TREATMENT OF THE ELDERLY
The prevalence of epilepsy increases sharply in the elderly. Common causes of seizures are cerebrovascular disease, degenerative dementia, and neoplasms in that order of frequency. Seizures are nearly exclusively partial onset, being simple partial, complex partial, or secondarily generalized in type. The postictal period is often longer in the elderly and may last several days after a generalized tonic–clonic seizure. EEG shows epileptiform abnormalities in 40% patients.
A. Major problems. Elderly patients with epilepsy constitute a special patient population because of age-related changes in the pharmacokinetics of AEDs, comorbidity, and multiple drug therapy. There is a decrease in plasma albumin with resultant overall decrease in protein binding of AEDs; hence, total concentration of highly bound AEDs (valproic acid, phenytoin, and so on) becomes misleading. Hepatic drug metabolism is decreased, and there is a steady decline in glomerular filtration rate, decreased tubular secretory function, and decreased renal blood flow with normal aging. These changes lead to longer elimination half-life and reduced clearance of many AEDs, with either primarily hepatic or renal elimination. In addition, concomitant medical problems and, consequently, multiple comedications further affect the absorption, disposition, and metabolism of AEDs. Elderly patients also tend to experience more side effects of AEDs, especially somnolence, confusion, gait disturbances, and postural and other tremors.
B. Management guidelines for using AEDs to treat the elderly.
1. AEDs should be prescribed only when the diagnosis of epileptic seizures is firmly established and there is a high probability of recurrence.
2. AED therapy should be initiated at lower doses and titrated more slowly to a daily dose that is on average lower by 30% to 40% than used to treat younger adults.
3. For high-protein-bound AEDs, measurement of free rather than total concentration may be more useful in evaluating seizure control or drug-related toxicity.
4. “Therapeutic ranges” of the traditional AEDs do not apply to the elderly mainly because of an overall decrease tolerability for drugs in this age group. Aim for a “low therapeutic” serum level, increasing the dose only if clinically necessary.
5. Carbamazepine, phenytoin, and valproic acid have been commonly used in the past (and still used in the developing countries) because the seizures are usually focal-onset, but their pharmacokinetics makes them less ideal for the elderly. The newer AEDs should be considered early in the treatment of the elderly patient because of their fewer drug interactions, minimal or no protein binding, and predominant renal elimination. However, the dose has to be adjusted downward in the presence of renal impairment. Most of the new AEDs are approved as adjunct therapy except oxcarbazepine and eslicabazepine, which may not be well tolerated in elderly because of neurotoxicity. Lamotrigine (presently approved for monotherapy conversion) has only minor interactions and good tolerability; hence, it is another alternative among newer AEDs. In several studies, levetiracetam has been noted to have few adverse effects and many experts use it (off label) as monotherapy in elderly. It is approved as monotherapy in European Union. It appears that levetiracetam, lamotrigine, and also gabapentin may emerge as common monotherapy AEDs for elderly patients with epilepsy.
DISCONTINUATION OF ANTIEPILEPTIC DRUGS
The decision to discontinue AEDs in a patient who has been seizure-free for several years depends on many prognostic factors. The overall relapse rate after AED withdrawal is approximately 30% to 40% among adults with chronic epilepsy.
A. Prognostic factors.
1. Types of epilepsy.
a. Some childhood forms of epilepsy (e.g., benign Rolandic epilepsy and childhood absence epilepsy) usually remit during adolescence.
b. Patients with PGE with onset in adolescence or adulthood have a good prognosis. Many remit, but some (especially JME) need lifelong therapy.
c. Adult patients with focal epilepsy, especially with complex partial seizures due to mesial temporal sclerosis, have a high recurrence rate (40% to 50%) after discontinuing AEDs.
d. Patients in whom seizures occur in the setting of an acute cerebral insult (e.g., trauma, infection, or stroke) may not have chronic epilepsy. Such patients should be considered for withdrawal of AED after a seizure-free period of 6 months.
2. EEG findings obtained just before discontinuation of AED therapy are useful predictors of the outcome. The relapse rate is four to five times higher if EEG shows persistent epileptiform abnormalities. In the presence of these abnormalities, most physicians consider continuing AEDs.
3. Other predictors of outcome. A high frequency of seizures, a long duration before seizures are controlled with AEDs, and multiple seizure types in a patient carry a less favorable prognosis. Similarly, patients who have structural abnormalities responsible for seizures or who have mental retardation or neurologic deficits are more likely to have recurrence of seizures after discontinuation of AEDs.
B. General guide lines for withdrawing AEDs.
1. The decision to wean AED in a patient who has been seizure free has to be individualized and undertaken with the patient’s full understanding of risks and benefits. There is no consensus as to how long should a patient be seizure free before AEDs could be withdrawn. In general, discontinuation of AEDs may be considered by the physician if the patient has been seizure-free for 2 to 5 years while taking AEDs, has normal neurologic examination, and normalized EEG with treatment.
2. Adult patients with PGE who have remained seizure-free for 2 to 5 years and whose EEGs show no paroxysmal abnormalities or photoparoxysmal response are good candidates for withdrawal of therapy unless they have JME.
3. Women who want to bear children and who have been seizure-free for several years should be considered for withdrawal of medication before conception to avoid possible ill-effects on the fetus.
4. However, most adults with focal epilepsy, secondary generalized epilepsy, or PGE manifesting as multiple seizure types probably need long-term AED therapy.
C. AED withdrawal mode and precautions.
1. AEDs must be withdrawn slowly, typically over 3 months or longer, especially in the case of barbiturates and benzodiazepines.
2. Patients taking more than one AED should have the less or least effective drug withdrawn before the first-line drug. Only after the patient has remained seizure-free for several months only on one AED, is the final drug withdrawn.
3. During the withdrawal period, the patient is advised to follow a restricted lifestyle (no driving or hazardous occupational or recreational activities) to minimize the consequences should the seizures recur.
4. If the seizures recur, the patient is promptly placed on an adequate dosage of an appropriate AED, which then probably has to be continued on a lifelong basis.
5. After the withdrawal period, attention has to be paid to such lifestyle issues as getting adequate sleep, avoiding alcohol, and avoiding stresses that can cause recurrence.
6. Epilepsy is considered to be resolved for individuals who had age-dependent epilepsy syndrome, but are now past the applicable age or those who have remained seizure free for the last 10 years with no seizure medication for last 5 years (ILAE, 2014).
SUDDEN UNEXPECTED DEATH IN EPILEPSY
The risk of dying is 3 to 4 times in patients with epilepsy and having a sudden death is about 24 times more likely compared to the general population. Increased mortality is because of multiple causes, for example, suicide, SE related to nonadherence to AEDs, and seizure-related physical injuries. In recent years, sudden unexpected death in epilepsy (SUDEP) has been recognized as an important concern in patients with epilepsy. SUDEP is defined as sudden and unexpected death in a patient with epilepsy without an obvious cause found at autopsy. At least one-sixth of all deaths in epilepsy patients represent SUDEP, risk being 5 to 7 times higher with medically refractory epilepsy.
Incidence of SUDEP is about 1 death per 1,000 epilepsy patients per year, perhaps an underestimation. The incidence rises to 1 death per 100 to 150 epilepsy patients per year in uncontrolled epilepsy.
Risk factors for SUDEP include uncontrolled and frequent seizures, especially of generalized tonic–clonic type, low AED levels, polytherapy, early onset and long duration of epilepsy, a seizure prior to the death, young adult age group (20 to 40 years), and death at night or during sleep in a prone position. The most important risk factor is the noncompliance with AEDs. SUDEP may be because of seizure-related cardiac and/or pulmonary causes. Epilepsy-related higher mortality and SUDEP are issues, which are generally ignored during patients’ office visit. These issues need to be discussed at least in those patients with longstanding and poorly controlled epilepsy not only to educate the patient and the family members of the potential for this fatal complication but also to strongly emphasize the need for AED compliance.
REFERRAL FOR COMPREHENSIVE TREATMENT
A. Psychosocial problems. Patients with epilepsy face many personal and psychosocial difficulties that require psychiatric referral and counseling. There is a high incidence of mood disorder; depression occurs in 20% to 50% patients. Citalopram and sertraline are preferred antidepressants to treat coexisting depression. These have no drug–drug interaction. Bupropion should be avoided because of its high potential in lowering threshold for seizures. Patients who need comprehensive care should be referred to a comprehensive epilepsy center equipped with multidisciplinary teams capable of providing psychosocial and vocational counseling in addition to the appropriate medical therapy.
B. Experimental AEDs. Approximately 20% to 30% of patients with epilepsy who have medically intractable seizures may be helped by referral to a center, conducting ongoing clinical trials of new AEDs. Such patients often need combination therapy with two or more AEDs, or some type of surgical treatment that are best handled in a comprehensive epilepsy center.
C. Surgical treatment.
1. Resective surgery. Epilepsy is considered medically refractory when seizure control is unsatisfactory despite trials of two or three AEDs, in monotherapy or in combination. After multimodality presurgical evaluation, if found to have a single epileptogenic focus that does not involve eloquent cortex, these patients may be candidates for resective surgery.
a. Anterior temporal lobectomy is the most frequently performed resective surgery when the seizures arise from anteromedial area or the lateral cortical area of the temporal lobe on one side. Epileptic patients with mesial temporal sclerosis on the MRI are excellent candidates because they are usually medically refractory but respond very well to surgery, over two-thirds rendered seizure-free.
b. Extratemporal resection for epileptogenic foci outside the temporal lobe is increasingly performed, although the success rate is 50% or less. Localizing an extratemporal seizure focus, for example, in the frontal lobe, is more challenging (may require invasive monitoring with subdural electrodes) unless a structural lesion is present on the MRI scan.
c. Corpus callosotomy is performed in a few epilepsy centers for patients with secondarily generalized epilepsy who suffer from frequent drop attacks. The procedure is followed by a number of complications such as disconnection syndrome, difficulty with motor and language function, and maintenance. Although drop attacks and generalized seizures respond favorably, rarely do seizures totally remit.
d. Hemispherectomy is reserved for a selected few patients (mainly children) who have unilateral hemispheric insult (Rasmussen’s encephalitis, Sturge–Weber syndrome, extensive cerebral dysgenesis, hemimegalencephaly, and so on), resulting in medically refractory focal epilepsy and hemiparesis.
e. Subpial transection is a surgical technique to treat focal epilepsy when the seizures arise from eloquent cortex such as the language or motor area. The procedure cuts horizontally connecting fibers so as to decrease propagation of seizure activity without resulting neurologic defects. The procedure is often combined with corticectomy.
2. VNS. The FDA has approved VNS as adjunctive therapy for medically refractory seizures in adults and children. VNS is considered for patients with focal or generalized epilepsy who have undergone unsuccessful polytherapy trials of several AEDs, including newer AEDs, who are not candidates for resective surgery or have undergone unsuccessful resective surgery.
VNS requires implantation of a programmable signal generator subcutaneously in the upper chest on the left side. This device is capable of delivering intermittent stimulation to the left vagus nerve in the neck by means of bipolar electrodes at the desired settings. In addition, the patient or a companion can activate the generator by placing the accompanying magnet over the generator for several seconds to interrupt a seizure or reduce its severity if administered at seizure onset.
The mechanism of action of VNS is unknown, but several large trials showed that approximately 30% to 40% of patients with refractory partial-onset seizures had a 50% or greater reduction in seizure frequency. Only a few had complete control of seizures. Overall, VNS has the same efficacy as many of the newer AEDs but without drug-related side effects. Patients with aurae are particularly helped because they can activate the device to abort an impending seizure. The common side effects include hoarseness, throat pain, coughing, dyspnea, and muscle pain. Possible surgical complications of hematoma, wound infection, left vocal cord paralysis, and injury to the vagus nerve or carotid sheath are rare when the procedure is performed by an experienced surgeon.
3. Neuropace, a responsive neurostimulator (RNS), has been recently (2014) approved by the FDA. It consists of placing depth or subdural electrodes in close proximity to the epileptogenic focus identified by prior presurgical evaluation. The RNS system works by detecting a seizure and then delivering an electric stimulation to abort the electrographic seizure. It is indicated in those medically refractory patients who are not candidates for resective surgery because they have either bilateral epileptogenic foci or a focus in or close to the eloquent cortex. The identification of the appropriate candidates requires surface and/or invasive monitoring by an expert team of epileptologist and surgeons in the level 4 comprehensive epilepsy center. Approximately 40% reduction in the seizures is achieved by 3 months.
4. Future trends. Bilateral stimulation of the anterior thalamic nucleus (deep brain stimulation) by placing multicontact depth electrodes stereotactically has been used in medically refractory epilepsy. Protocol-driven studies have shown a 39% median reduction of seizures in the treatment group compared to 23% in the controlled group.
Key Points
• Evaluation of a patient suspect with epilepsy should include a detailed history of episodes, MRI of the brain, and EEG studies with the goal of not only confirming that the episodes are epileptic seizures, but also defining the epilepsy syndrome.
• Patients with epilepsy, who fail to respond to two or more appropriate AEDs in monotherapy or combined therapy, are considered medically refractory. They need to be referred to comprehensive epilepsy center for further evaluation and management, which may include resective surgery, neurostimulation, or experimental medication.
• Patients with symptomatic localization-related epilepsy because of mesial temporal sclerosis are very likely to remain medically refractory and best treated by surgical resection.
• Young women with epilepsy need to be treated with AEDs with least incidence of major fetal malformation and using monotherapy in least effective dose. Appropriate AED selection needs to be planned before pregnancy and the women need to be followed closely during pregnancy to adjust doses of the medication according to the blood levels, which commonly decrease during pregnancy.
• Medically refractory patients with epilepsy, who have frequent seizures, particularly of generalized tonic–clonic type, are at increased risk for SUDEP. The incidence of this tragedy can be reduced by a rigid compliance to AEDs and reducing the frequency of seizures by more aggressive control of seizures by all options including surgical treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree