Fig. 39.1
Video electroencephalogram (EEG): ictal EEG showing rhythmical theta activity in electrodes in the right mesial temporal lobe
Over recent years, improvements in imaging techniques such as magnetic resonance imaging (MRI) have represented a major advance, permitting a precise evaluation of the changes mainly located in the hippocampus. MTS is characterized by atrophy of this structure in the T1-weighted sequence or in the volumetric reconstructions and by an increase of the signal in T2-weighted and the fluid-attenuated inversion recovery sequence (Fig. 39.2) [9].
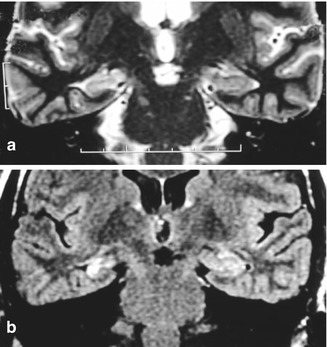

Fig. 39.2
Right mesial temporal sclerosis. (a) Hippocampal atrophy on T1-weighted imaging. (b) Signal increase in fluid-attenuated inversion recovery sequence
In patients with MTS, the congruence of these data (clinical, interictal EEG, VEEG, SPECT, and MRI) suggests a better prognosis for surgical treatment. When doubts exist, especially regarding the lateralization of the seizures, invasive evaluation can be used by bilateral implantation of deep electrodes into the hippocampus.
Surgery is the recommended treatment. Randomized studies have demonstrated that after 1 year, seizure control is at least twice as efficient in patients who submit to surgical treatment as in patients receiving medicamentous treatment [59].
Surgical treatment of MTS is based on two interventions: anterior temporal lobectomy and selective amygdalohippocampectomy sparing resection of the temporal neocortex. No statistical difference has been demonstrated between these two methods regarding the efficiency of seizure control or the prevention of cognitive disorders [31].
Anterior temporal lobectomy starts with resection of the temporal neocortex including the middle and inferior temporal gyri, permitting a better view of the mesial structures. The posterior extension differs according to the side: about 5–6 cm in the nondominant hemisphere and 3–4 cm in the dominant hemisphere [51]. Using a subpial technique, the entire lateral temporal parenchyma and uncus are removed up to the limit consisting of the medial margin of the tent. This permits the exposure laterally through the pia mater, of cranial nerve III and the posterior cerebral artery, and of the internal carotid, posterior communicating, and choroidal arteries medially. This resection in a posterior direction creates a space that permits the lateral retraction of the hippocampus, facilitating the coagulation of its hilus. The next step is the opening of the ventricular cavity in order to expose the amygdala and the hippocampus. To facilitate the location of these structures, a corticectomy is performed from the superior temporal sulcus, 3 cm posterior to the tip of the temporal lobe, in the direction of the free margin of the previously exposed tent. The amygdala is the first structure to be removed using, as a reference, the upper limit of the line formed between the emergence of the choroidal artery into the internal carotid artery and its entry into the choroidal fissure of the temporal horn (choroidal point). This limit is important in order to prevent damage to the optic tract [58]. Resection of the hippocampus starts from the anterior portion (head) and extends posteriorly through its medial surface, with coagulation of the hilus and of the branches originating from the posterior cerebral artery. The hippocampus is removed in a block of 2.5–3 cm up to the posterior portion of the cauda, permitting the complementary removal of the parahippocampal gyrus in its more medial portion (Figs. 39.3 and 39.4).
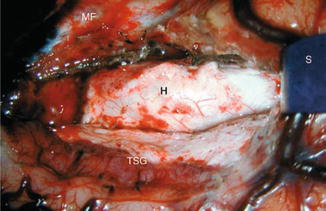
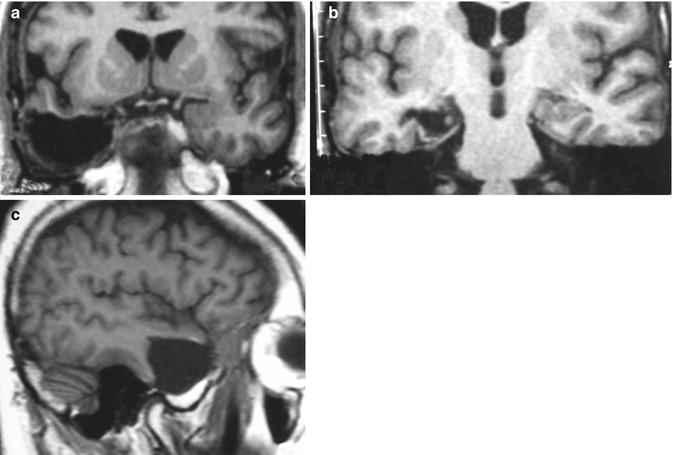

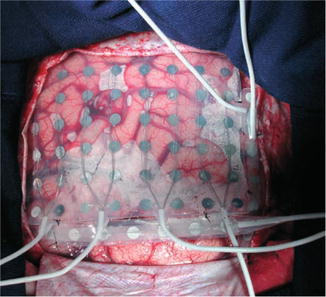
Fig. 39.3
Surgical exposure of right hippocampus (H). TSG temporal superior gyrus, MF medial fossa, S spatula

Fig. 39.4
Postoperative magnetic resonance imaging (MRI) showing resection of the right neocortex (c), amygdala (a), and hippocampus (b)
Regarding the results of anterior temporal lobectomy, 65–80 % of patients have been reported to remain seizure-free. This difference is due to different criteria for the evaluation of the results and to refractoriness for surgical indication. In our series of 60 patients with temporal lobe epilepsy followed up for at least 2 years, 69 % showed results compatible with Engel Ia or Ib [12]. However, if we consider only patients with MTS, this rate reaches 80 % (Fig. 39.5).
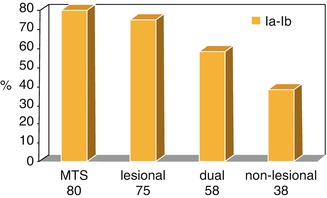

Fig. 39.5
Results in temporal lobe surgery. Percentage of patients with Engel Ia–Ib results. MTS mesial temporal sclerosis, Dual MST, and lesional
Several complications have been reported regarding this procedure: superior quadrantopsy, hemianopsia, hemiplegia due to lesion of the choroidal artery, and memory and speech disorders. In our series we observed a patient with late osteomyelitis, a patient with temporary dysfunction of cranial nerve III, and two asymptomatic patients with a chronic subdural hematoma revealed by postoperative MRI.
39.4 Extratemporal Epilepsies
While about 80 % of patients with temporal epilepsy present with an anatomical substrate (hippocampal sclerosis), the extratemporal epilepsies are heterogeneous in terms of their etiology. Recent advances in imaging examinations have led to a rapid increase in the number of identifiable lesions, especially cortical dysplasias, which play an important role in the genesis of extratemporal epilepsies. However, approximately 50 % of refractory extratemporal epilepsies are not definitely identified by neuroimaging studies [52].
Historically, the first surgeries for extratemporal epilepsies date back to the work of Rasmussen and Penfield at the Neurological Institute of Montreal [44, 45, 47]. These investigators classified the pre- and postcentral gyri as a specific region, denoted the central area. On this basis, surgical epilepsies were identified as being 56 % temporal, 18 % frontal, 7 % in the central region, 6 % in the parietal lobe, and 1 % in the frontal lobe [45]. Multilobar resections or hemispherectomy was used to treat 11 % of the patients. Other series focusing on surgical treatment of extratemporal epilepsies identified 45–64 % of the surgeries as being performed after seizures starting in the frontal lobe, 7–13 % starting in the parietal lobes, 2–23 % as being occipital, and 23–44 % as multilobar foci [25]. In the surgical series published by Eriksson et al. [13], while 75 % of adult surgeries involved the temporal lobe, only 25 % of children’s surgeries involved this lobe.
The semiology of extratemporal syndrome is varied and is not well characterized, even when limited to a single lobe. Extratemporal epilepsies also tend to spread rapidly, impairing their location on the basis of clinical characteristics. In some cases, especially those involving the frontal lobe, the seizures rapidly cross to the contralateral side, also impairing their lateralization [38].
The presence of a focal finding in MRI is probably the most important factor in the approach to extratemporal epilepsies, since a focal lesion classifies the epilepsy as lesional, with a better surgical prognosis. Other imaging studies such as ictal or interictal SPECT, PET, and spectroscopy can be of help for the focal diagnosis when MRI is normal [36].
Most patients with extratemporal epilepsies refractory to medicamentous treatment present extensive irritative multilobar surface areas in their EEG monitoring [36, 38]. In a series of 30 patients with localized forms of cortical dysplasia, more than half of the subjects presented a greater distribution of interictal findings than the structural lesion, with two-thirds of them being multilobar [35]. The identification of lobar distribution, in turn, is insufficient for a precise topographic definition. In addition, extensive neocortical areas located in the interhemispheric and basal regions, and therefore “far” from the surface electrodes, may be initial firing foci. These factors, together with the property of extratemporal foci, especially of the frontal lobe, to rapidly propagate also to the contralateral hemisphere, lead to an inconclusive, or even “false-positive,” location of the epileptogenic foci [24]. Thus, it is necessary, especially when the structural lesion cannot be located, to use invasive monitoring (i.e., the placement of deep electrodes in the cerebral parenchyma or subdural electrodes on the cortical surface) [35].
Deep electrodes are fine cables with cylindrical contacts along their terminal extremity, which are placed inside the encephalic parenchyma. They are used when there is a suspicion of epileptogenic areas deeply located or, more commonly, in patients with temporal epilepsy who cannot be correctly lateralized due to the rapid propagation of the impulse to the contralateral temporal lobe. They are commonly implanted stereotaxically by trephining along the direction of the hippocampus (entering through the occipital lobe) or orthogonal to the axis of the hippocampus (entering through the temporal lobe; Fig. 39.6).

Fig. 39.6
Deep bilateral temporal electrodes
The need for implantation involves a 1–4 % risk of infection and a risk of cerebral hemorrhage of 3 % in parasagittal placement and of 1 % in lateral placement [42]. The placement of bilateral intrahippocampal electrodes may be associated with a postoperative decline of verbal memory [41].
Subdural electrodes are used to map the surface of the encephalon and to delimit the region of seizure onset in the neocortex. The electrodes are fine platinum or stainless steel disks attached to a fine plastic surface arranged in the configuration of striae or plates. They are placed in intimate contact with the cerebral parenchyma by craniotomy, and they can be placed in mesial structures. Subdural electrodes are used not only for ictal recording and for the determination of the zone of seizure onset but also for cortical stimulation and mapping of cortical function on this area [52]. Six cases of infection (two cases of meningitis, one abscess, and three infections of the surgical wound) were reported in a series of 350 patients. In another series of 131 patients, 2.5 % of them presented small hematomas that did not require surgical drainage [41]. Analyses of a limited amount of cortical surface and enhancement or reduction of the epileptiform activity over the underlying cortex are disadvantages of this method (Figs. 39.7, 39.8, 39.9, 39.10, and 39.11) [7].
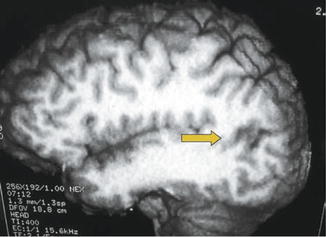
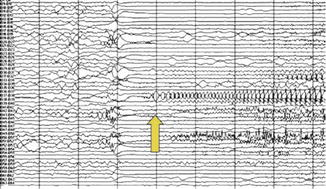
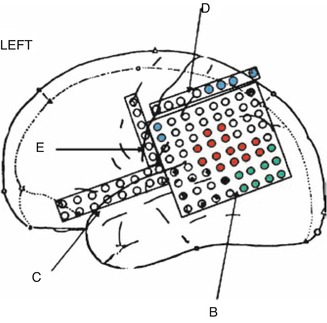
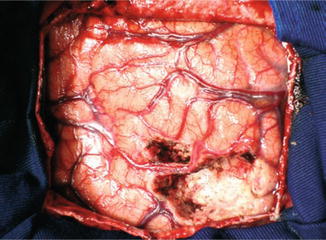

Fig. 39.7
Gliotic area near the left angular gyrus (arrow) in a 40-year-old man with partial seizures

Fig. 39.9
Ictal EEG recording during a partial seizure from the patient of Fig. 39.7. Repetitive spikes in contacts B38, 42, 51 corresponding to the area surrounding the language area. The arrow marks the electrographic seizure onset

Fig. 39.10
Results of electrical stimulation of the cortex from the patient in Fig. 39.7 with subdural electrodes in the left hemisphere. Key: red ictal zone, green visual area, blue sensitive area, and black and white language area

Fig. 39.11
Postresection with preservation of the arterial supply for the adjacent cortex from the patient in Fig. 39.7
Eloquent areas such as the motor cortex, sensitive cortex, language areas (Broca’s area, Wernicke’s area, supplementary motor area, basal temporal area), and visual areas can be identified by cortical stimulation either in the operating theater (motor and language) or during the period of invasive VEEG evaluation when subdural electrodes are implanted.
The interictal epileptiform activity can be recorded directly from the cerebral cortex during surgery (electrocorticography) and is considered to be an indispensable technique for defining the irritative zone in the intraoperative evaluation. During the subdural grid implantation, it is useful for better defining the areas of the cerebral cortex to be evaluated. After electrode removal and cortex resection, it is a tool for determining any residual epileptiform activity.
Patients with extratemporal epilepsy who submit to surgical treatment are divided into two major groups: patients with lesional epilepsies and patients with cryptogenic epilepsies whose imaging exams do not identify lesions. Patients with lesional epilepsies have a better prognosis regarding seizure control. In a representative study of 60 patients with extratemporal epilepsy, 61 % of the patients with identified structural lesions were seizure-free over a period of 5 years, as opposed to 20 % of nonlesional patients [60]. In a review of frontal epilepsies from 1987 to 1994, 72 % of the lesional patients had adequate seizure control (Engel I or II), after 5 years, as compared to 40 % of nonlesional patients [29].
39.4.1 Lesional Epilepsies
Of patients with refractory extratemporal epilepsies, 50 % present identifiable structural lesions, representing up to 80 % of patients in the surgical series [36]. Focal abnormalities such as tumors, vascular lesions, and cortical abnormalities show different degrees of differentiation from the parenchyma, a fact that influences the surgical programming [25].
Neoplasias such as low-grade astrocytomas, oligodendrogliomas, dysembryoplastic neuroepithelial tumors, and others may often be the origin of epileptic foci (Figs. 39.12, 39.13, and 39.14). Epileptogenic tumors usually grow slowly, occur in young individuals, and involve the gray matter. Epileptogenic control is better when resection of the entire lesion and the marginal area (extended lesionectomy) is possible, with a rate of seizure control of about 80 % [25].
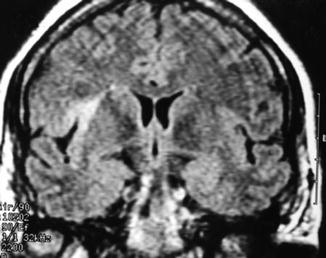

Fig. 39.12
A 35-year-old woman with left frontal granuloma

Fig. 39.13
A 13-year-old boy with a right frontal dysembryoplastic neuroepithelial tumor

Fig. 39.14
A 51-year-old woman with a right insular dysplasia
Cavernomas and arteriovenous malformations are vascular lesions commonly associated with epileptic seizures [25]. Lesionectomy with the removal of marginal areas by impregnation with hemosiderin leads to seizure control in 73 % of patients [22].
As diagnostic imaging methods evolve with increasing sensitivity, anomalies of cortical development are becoming increasingly more frequent as the cause of focal epilepsy. The most common developmental anomaly is cortical dysplasia, followed by malformations of the cortical gyri such as polymicrogyria. Developmental lesions are observed more frequently in children, often involving large extensions of the cortex [13]. Cortical dysplasias are associated with a particular pattern of epileptogenicity, with some authors believing that intraoperative electrocorticography is a necessary tool [37]. In large series, 49 % of the patients with dysplasia became seizure-free, with 58 % having submitted to complete resection and 27 % to incomplete resection. Cortical dysplasia with Taylor’s balloon cells was completely removed with a 100 % success rate in a series of 16 patients [41].
39.4.2 Nonlesional Epilepsies
Patients with nonlesional epilepsy represent the greatest challenge for the surgical treatment of epilepsies, with a lower success rate ranging from 20 to 55 % of the patients. The use of invasive preoperative electrocorticography appears to play a fundamental role in this type of patient. A study conducted on 24 patients with epilepsy and normal MRI did not reveal a significant difference in seizure-free evolution in patients submitted to invasive monitoring. However, the use of invasive monitoring was of fundamental importance for the surgical strategy by establishing a clear definition between the epileptogenic zone and the motor and language areas [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree