Ethosuximide
Edward B. Bromfield
Introduction
Among several succinimides synthesized as modifications of the hydantoin-barbiturate heterocyclic ring, only one, ethosuximide, is still in widespread use; another, methsuximide, has a broader spectrum of action but is used less often (see Chapter 163). Ethosuximide has been available since 1958, and remains a first-line drug for the treatment of absence and related seizures.
Chemical Structure, Formulations, and Methods for Determination in Body Fluids
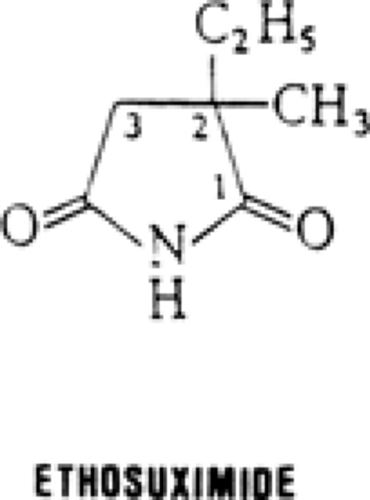
The similarity of ethosuximide, 2-ethyl-2-methylsuccinimide (Fig. 1), to other traditional antiepileptic drugs (AEDs) with a five- or six-membered heterocyclic ring structure is apparent, with a nitrogen atom located between two carbonyl groups. Unlike most, however, it is highly water-soluble. It has one asymmetric carbon and is used in its racemic form.22 It is a crystalline powder or a waxy solid, sold as a liquid-filled capsule (250 mg) or as syrup (250 mg/5 mL). High-performance liquid chromatography can be used to measure plasma levels.28
Pharmacology
Activity in Experimental Models of Seizures/Epilepsy
Ethosuximide’s efficacy against absence seizures is consistent with its potency in several chemical convulsant models, particularly pentylenetetrazol, as opposed to the maximal electroshock model.14 It is also successful in other models believed to mimic absence seizures, including systemic penicillin, intravenous γ-hydroxybutyrate, intraventricular leucine-enkephalin, and spontaneous seizures in selected genetically epilepsy-prone rodents (Strasbourg), and as well experimental seizures induced by fluorothyl, enflurane, barbiturate withdrawal, and cortical estrogen or cobalt.14,19,21,25 On the other hand, ethosuximide is relatively ineffective against seizures induced by bicuculline, strychnine, aminophylline, kainic acid, and N-methyl-D-aspartate, as well as cortical aluminum or amygdaloid kindling.14,21,25
Activity in Other ModelszHH
Low-threshold, “transient” (T-type) calcium (Ca) channels on thalamic neurons seem to play a critical role in the thalamocortical interactions that underlie the 3-Hz spike-and-wave pattern characteristic of absence seizures.14,19 Ethosuximide blocks the low-threshold Ca current mediated by these channels in a voltage-dependent manner, such that the degree of current blockade increases at more hyperpolarized conditions.8,14,25 That this mechanism is adequate to decrease abnormal thalamocortical excitability without affecting normal, tonic thalamic cell activity has been demonstrated in a computer simulation.17 Dimethadione, another specific antiabsence drug, also blocks these currents at therapeutically relevant concentrations, whereas carbamazepine and phenytoin do not,8,19 and valproate does so to a much lesser degree.19 Ethosuximide also has other potentially important ionic effects, such as partial blockade of noninactivating sodium (Na) currents and Ca-dependent potassium (K) channels.14,16
Mechanisms of Action
Although the main effect of Ca-current blockade appears to be a decrease in thalamocortical excitation, ethosuximide may also interfere with rhythmic inhibitory activity,11,14 which appears to be important in generating absence seizures.2 Effects on neurotransmitter synthesis, metabolism, or action do not seem to underlie these effects, but the effects on T-type Ca channels discussed earlier could be responsible.8,14
Clinical Pharmacokinetics
Absorption
Absorption after oral administration is essentially complete, and peaks 3 to 5 hours after ingestion of the capsule;22 absorption occurs slightly more quickly in acute than in long-term administration, and with the syrup as opposed to the capsule formulation. No significant first-pass metabolism occurs.
Plasma Protein Binding and Distribution
Because of ethosuximide’s high water solubility, its volume of distribution is high, estimated at 0.7 L/kg.22 Concentrations in adipose tissue are about one-third of those in plasma.22 Ethosuximide rapidly crosses the blood–brain barrier, and equilibration between plasma and cerebrospinal fluid occurs in 20 to 30 minutes; this is three to four times as rapid as for valproate, phenobarbital, phenytoin, or carbamazepine.22 Concentration is uniform within the brain. Because ethosuximide is not significantly protein-bound, cerebrospinal, salivary, and lacrimal concentrations are similar to those of plasma. Breast milk has only slightly lower concentrations, with a milk-to-plasma ratio of 0.8 to 0.9.15,22
Metabolism and Excretion
During long-term administration, approximately 20% of the dose is excreted renally unchanged, with the remainder
hepatically metabolized to inactive compounds via the cytochrome P450/mixed-function oxidase system, principally CYP3A.1,22 These reactions include mainly hydroxylation of the ethyl side chain in either the 1- or 2- position (see Fig. 1), with metabolites conjugated predominantly with glucuronic acid and then excreted in the urine. Elimination of ethosuximide follows first-order kinetics.22 In adults, serum half-life is approximately 40 to 60 hours, with substantial variation between individuals; in children and newborns, half-life is slightly shorter, 30 to 40 hours.15,22 Levels during pregnancy may fall, but not as much as those of some other drugs.15,22 Although autoinduction of metabolism has been demonstrated in rats, this process probably does not occur in humans.22
hepatically metabolized to inactive compounds via the cytochrome P450/mixed-function oxidase system, principally CYP3A.1,22 These reactions include mainly hydroxylation of the ethyl side chain in either the 1- or 2- position (see Fig. 1), with metabolites conjugated predominantly with glucuronic acid and then excreted in the urine. Elimination of ethosuximide follows first-order kinetics.22 In adults, serum half-life is approximately 40 to 60 hours, with substantial variation between individuals; in children and newborns, half-life is slightly shorter, 30 to 40 hours.15,22 Levels during pregnancy may fall, but not as much as those of some other drugs.15,22 Although autoinduction of metabolism has been demonstrated in rats, this process probably does not occur in humans.22
There is little data on ethosuximide concentrations in hepatic or renal disease, but increases would be expected. Ethosuximide is dialyzable, with an estimate of a 50% decrease in levels over a 6-hour dialysis session.22
Relationship Between Plasma Concentrations and Effects
Given ethosuximide’s relatively long half-life, it takes 7 to 12 days of administration to reach steady-state plasma levels, and little variation is noted between peaks and troughs, even with once-daily administration,22 although it is usually administered twice a day. Average daily doses are 15 to 40 mg/kg to achieve the usual therapeutic range of 40 to 100 μg/mL. Although the dose-level relationship is generally linear within the usual dose range, there is evidence of saturation kinetics in some patients.22 Among 33 patients with complete control of absence seizures, 91% had levels of at least 40 μg/mL.29