CHAPTER 56 Evaluation of Patients for Epilepsy Surgery
Up to 40% of epilepsy patients have medication-resistant or medically intractable seizures.1 As many as half of these patients may be candidates for curative or palliative epilepsy surgery. After successful epilepsy surgery, quality of life has been shown to improve significantly.2 The degree of success of epilepsy surgery depends largely on careful presurgical evaluation for identification and selection of the most appropriate candidates. The evaluation usually takes place in centers with a dedicated multidisciplinary team of neurologists and neurosurgeons experienced in treating patients with epilepsy, supported by electroencephalographers, neuroradiologists, neuropsychologists, psychiatrists, and paramedical staff. The aim of this chapter is to review the key elements of the presurgical evaluation and to outline some of the principles used in the decision-making process for identification of appropriate candidates for epilepsy surgery.
Initial Considerations in Choosing a Candidate for Presurgical Evaluation
We apply three fundamental criteria to identify candidates for epilepsy surgery:
In evaluating patients according to these three overarching criteria, it is important to note that intractable epilepsy is a progressive disorder that is medically, physically, and socially disabling.3 Accidental injuries as a result of seizures, psychiatric morbidity, loss of independence attributable, in part, to the inability to drive, and chronic medication toxicity all have major impacts on the quality of life of affected individuals. Recognition of this situation has gradually led to earlier consideration of epilepsy surgery, although in many series the duration of refractory epilepsy before surgery still exceeds 10 to 15 years.4
Epilepsy surgery may be proposed as a curative treatment or as a palliative treatment. Curative procedures, aimed at focal epilepsy, are almost always resective and require clear and complete identification of the epileptogenic zone (EZ). The majority of epilepsy resections target the anterior temporal lobe, including the mesial structures, and are associated with a high rate of remission. By contrast, extratemporal foci, most commonly frontal, may present additional difficulties because the EZ may be difficult to fully delineate and may involve eloquent cortex. With high-resolution neuroimaging techniques and dedicated imaging protocols, extratemporal lesions such as small heterotopias, cavernous angiomas, and focal cortical dysplasias can be visualized in up to 50% of patients, thereby dramatically improving their chance for success after epilepsy surgery. Rates of long-term freedom from seizures after resection of extratemporal lesions have improved but may still be lower than those for temporal lobe resection.5,6 In a recent meta-analysis, the median proportion of patients seizure free was 66% after temporal lobe resection, 46% after occipital or parietal resection, and 27% with frontal lobe resection.7
Palliative techniques include disconnection procedures and multiple subpial transections. Corpus callosotomy, typically performed as a two-stage procedure, may be helpful for patients with secondary generalized epilepsy syndromes and drop attacks.8 The aim of this procedure is to primarily palliate patients with atonic seizures and improve their quality of life. This procedure may offer up to 35% freedom from the most disabling seizures.7 Multiple subpial transections are thought to predominantly sever horizontal connections in the cortex while preserving columnar organization and efferent pathways.9 This technique may be used in isolation for epileptic foci lying exclusively in eloquent cortex or as an adjunct along with partial resection to approach EZs that cannot be fully removed.
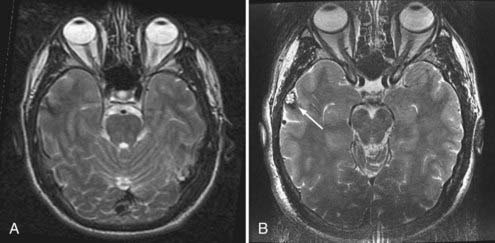
Evaluation of patients for consideration of epilepsy surgery includes a careful clinical history and physical examination, neuroimaging, and electroencephalographic (EEG) monitoring, often supplemented with detailed neuropsychological testing and psychiatric assessment when appropriate. The goal is to assess the concordance of multiple streams of data with respect to the probable EZ. Although the precise steps in the investigation vary somewhat from center to center, there is general consensus on the approach. Investigations are typically considered in phases. In general, phase I includes noninvasive recording, whereas phase II involves invasive intracranial EEG monitoring. Importantly, the investigation is an iterative process of hypothesis testing in which the results from each modality inform the interrogation of others. For example, identification of high-amplitude focal spikes on an EEG recording may prompt renewed scrutiny of magnetic resonance imaging (MRI) scans in the suspect area, thereby revealing a subtle developmental abnormality or vascular lesion (Fig. 56-1).
Phase I—Noninvasive Investigations
Routine Electroencephalographic Recording
Although the value of routine outpatient EEG studies in the decision-making process for consideration of epilepsy surgery is limited in comparison to that of other tests such as prolonged video-EEG monitoring and neuroimaging, its usefulness in the diagnosis of epilepsy and elucidation of the underlying syndrome is invaluable.10 Routine EEG findings are positive in 50% to 60% of patients with epilepsy, and the yield is increased by repeated or prolonged recordings that sample drowsiness and sleep. Even though focal slowing may signify an underlying structural abnormality, the major utility of routine EEG recordings is in the identification of interictal epileptiform discharges (IEDs) (sharp waves, spikes, or spike and wave complexes).11 Routine EEG studies aim to answer certain key questions: (1) Are there any IEDs present, and if so, how frequent are they? (2) Are the IEDs diagnostic of an idiopathic generalized syndrome that would render the patient inappropriate for resective epilepsy surgery? (3) If not, are the IEDs confined to one hemisphere or are they bilateral? (4) If the IEDs are unilateral, are they confined to one area of the hemisphere, such as the temporal region, or are they multifocal? The ideal surgical candidate would typically have a single population of well-localized IEDs, thus supporting the presence of only one EZ.
Continuous Video-Electroencephalographic Recording
Continuous computer-assisted video-EEG monitoring has become the mainstay of localization of the zone of epileptogenesis. The goals of video-EEG recording are threefold: (1) to further characterize the interictal EEG recording; (2) to detect, characterize, and quantify the patient’s habitual seizures12; and (3) to acquire physiologic data regarding seizure localization that can be correlated and compared with anatomic data obtained from neuroimaging studies. These investigations typically occur over a 5- to 7-day inpatient hospital stay (for adults) but are obviously dependent on seizure frequency and last as long as required to capture a satisfactory number of seizures.13,14 Characterizing the patient’s seizures is crucial because it allows (1) correlation of the ictal behavior with the electrographic discharge, (2) establishment of whether a patient has more than one seizure type, and (3) lateralization (which hemisphere) and localization (which area of the hemisphere) of the onset of seizures and therefore identification of the EZ. Typically, activating procedures such as sleep deprivation are used in addition to reduction or cessation of antiepileptic drugs in an attempt to increase the likelihood of seizure occurrence. In patients with nonlesional mesial temporal epilepsy, statistical analysis indicates that five concordant seizures will yield a 95% chance that the patient’s epilepsy is well lateralized (i.e., there is less than a 5% chance that five events would arise from the same side by chance alone).15 In addition to lateralization and localization, video-EEG recording of clinical seizures allows assessment of the temporal relationship between behavioral and electrical seizure onset. Prolonged delay before the first appearance of ictal EEG discharge should raise suspicion that the scalp recording may represent propagated activity from a remote site of onset.
Imaging of the Epileptogenic Zone
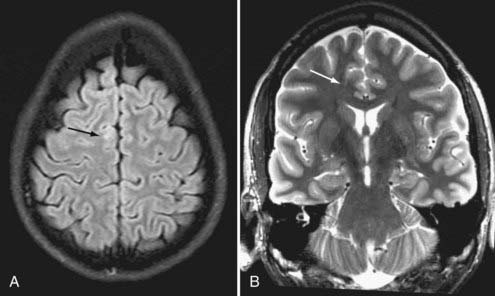
Brain MRI is the neuroimaging modality of choice for the presurgical evaluation of patients for epilepsy surgery. Hippocampal sclerosis, the most common pathology associated with temporal lobe epilepsy, is diagnosed on MRI by identification of all or some of the elements of volume loss, increased T2 signal, and loss of normal internal architecture in the affected hippocampus. Although fluid-attenuated inversion recovery (FLAIR) imaging makes signal abnormalities most obvious, true T2-weighted fast spin echo (FSE) sequences provide optimal signal-to-noise ratios for the examination of volume and architecture. In some centers, quantitative image analysis techniques may be used to calculate the volume of the hippocampi to detect subtle asymmetries16; however, useful deployment of these tools is labor and resource intensive and requires a high level of technical skill and experience. In addition to hippocampal sclerosis, specific MRI epilepsy protocols involving T1-, T2-, and susceptibility-weighted sequences are used to identify congenital and acquired epileptogenic lesions, most commonly low-grade gliomas, dysembryoplastic neuroepithelial tumors, malformations of cortical development (MCDs), cavernous angiomas, and subtle posttraumatic encephalomalacia and ulegyria. Many MCDs are subtle and best appreciated by looking for blurring of the gray-white junction on high-resolution MRI (Fig. 56-2). Excellent anatomic definition of the gray-white junction is now possible with phased-array surface coils deployed in magnets with high field strength, now up to 7 T.17
Functional imaging with positron emission tomography (PET) and single-photon emission computed tomography (SPECT) offers information about ictal or interictal brain tissue metabolism. PET using [18F]fluorodeoxyglucose (FDG) typically shows regional hypometabolism around the EZ when the tracer is injected interictally. PET was therefore a very useful tool for lateralization in patients with temporal lobe epilepsy or for identification of extratemporal EZs, such as MCDs,18 in the era before the development of high-resolution MRI scanning.19 Recently, however, with the increasing anatomic definition offered by MRI, the number of patients with partial epilepsy and seemingly normal MRI findings has decreased. As a result, the value of PET in this respect has also decreased. Nevertheless, it remains a useful tool for verification of MRI and EEG findings and in patients with normal findings on MRI. Other tracers such as [11C]-flumazenil show comparable results to FDG, but with a more restricted distribution of hypometabolism, which may be advantageous, particularly with extratemporal EZs.20
SPECT images are generated by photons emitted after the intravenous injection of a radioactive isotope such as technetium 99m-hexamethylpropyleneamine oxime (99mTc-HMPAO). The isotope is taken up by brain tissue in proportion to blood flow. Therefore, when injected within the first few seconds of a partial seizure, it shows increased uptake in the area of highest blood flow. This ictal SPECT is then compared with a baseline interictal study from the same patient to detect the differences in regional perfusion. This allows identification of the area hyperperfused at the time of seizure onset. The main disadvantage of ictal SPECT is that injection of the isotope has to be performed within the first few seconds after seizure onset, which is often impractical. The advantage, however, is that after injection, the window for scanning the patient is a few hours. SPECT can also be coregistered to MRI for better anatomic correlation of the EZ, and this can be used both for guidance of intracranial electrode placement and for subsequent surgical resection in a technique called subtraction ictal SPECT coregistered to MRI (SISCOM).21
Functional MRI (fMRI) images the blood oxygen level–dependent T2 signal (BOLD or T2*), a method whereby changes in metabolism and blood flow during an active process such as a simple motor or sensory task are detected. It has two roles in the identification of candidates for epilepsy surgery. fMRI is frequently used to map eloquent cortex during the surgical planning process. In this respect, fMRI aims to replace or limit the use of more invasive techniques such as the Wada test to identify language dominance and extraoperative or perioperative direct electrical brain stimulation to perform motor, sensory, and language mapping An emerging role for fMRI is in identification of the EZ. In this application, fMRI can be combined with simultaneous EEG recordings in an attempt to detect changes in blood flow and brain metabolism during an epileptic discharge.22 With improving technology, this could be a useful tool in the future for identifying the EZ, particularly in patients with normal findings on MRI.
Magnetoencephalography (MEG) is also emerging as an adjunctive, noninvasive tool for identification of the EZ. This technique measures the magnetic fields produced by electric currents in the brain, in this case epileptiform discharges. The fields generated are very weak, and hence very sensitive devices are required to detect them. Furthermore, the MEG scanner needs to be in a magnetically shielded room to eliminate outside magnetic signals. For these reasons, the device remains expensive and available only in research centers. Its main advantage is that although both MEG and EEG signals are generated by the same neurophysiologic processes, the MEG signal is not distorted by the skull and scalp. One additional advantage is that MEG is a good tool for noninvasive localization of the central sulcus with the use of somatosensory evoked magnetic fields.23 Overall, however, its value in surgical planning in relation to a seizure-free outcome has not yet been fully established.24
More recently, magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI) may offer additional clues to identification of the EZ in patients with MRI-negative partial epilepsy. MRS simultaneously detects and quantifies a number of brain metabolites and can therefore detect differences either between the two hippocampi, as in the case of hippocampal sclerosis, or between intralesional and perilesional tissue changes in MCD.25 In patients with hippocampal sclerosis, the typical finding in the epileptogenic hippocampus is a reduction in N– acetylaspartate and an elevation in choline-related compounds and creatine plus phosphocreatine (total creatine); the contralateral hippocampus may be normal or show a lesser degree of abnormality.26 Tractography, an important application of DTI, allows calculation of the direction of white matter tracts and may be helpful in determining the connections of the EZ to other brain areas, thus offering additional clues for identification of the EZ in patients with MRI-negative partial epilepsy.27,28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree