Fig. 1
Ex vivo delivery of growth factor s targeting skeletal muscles for ALS . A GFP-expressing hMSC line was genetically modified by lentiviral infection to stably release growth factors such as glial cell line-derived neurotrophic factor [10, 11]. These cells can be used for ex vivo gene therapy to deliver growth factors following intramuscular transplantation in a familial ALS rat model
The protocols in this chapter provide detailed information to expand and genetically modify hMSCs to release growth factor s by lentiviral infection. Detailed protocols for hMSC transplantation into the rat skeletal muscle are also described.
2 Materials
2.1 Human Mesenchymal Stem Cell (hMSC) Culture
1.
hMSCs: hMSCs are established using previously described methods (see Note 1 ) [12–14]. The hMSCs are derived from neonatal bone marrow aspirates from healthy donors after informed consent (see Note 2 ) and are transduced with a retroviral vector containing enhanced green fluorescence protein (GFP) using second passage hMSCs [12]. Aliquot hMSCs in cryovials and store in liquid nitrogen.
2.
hMSC culture medium: Dulbecco’s Modified Eagle Medium (DMEM high glucose, GlutaMAX™; Life Technologies, Carlsbad, CA, USA) supplemented with 20 % heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 50 U/ml penicillin, and 50 mg/ml streptomycin (see Note 3 ).
3.
0.05 % trypsin-EDTA (Life Technologies, Carlsbad, CA, USA).
4.
Dulbecco’s Phosphate-Buffered Saline (DPBS).
5.
24-well culture plates.
6.
T25-, T75-, or T175-culture flasks.
7.
15- or 50-ml conical tubes.
8.
0.6- or 1.5-ml microcentrifuge tubes.
9.
0.4 % trypan blue solution (Sigma-Aldrich, St. Louis, MO, USA).
10.
Hem☺ocytometer.
2.2 Genetic Modification of hMSCs by Lentiviral Vectors
1.
Lentivirus : Use a lentiviral construct for constitutive expression of human GDNF or VEGF -165 (VEGF) under the control of the mouse phosphoglycerate kinase 1 (pgk-1) promoter (see Note 4 and Fig. 2a) [10, 11, 15, 16]. Obtain high-titer stocks by ultracentrifugation, and suspend the lentiviral particles in 1 % bovine serum albumin (BSA) in phosphate buffered saline (PBS). Determine particle content of viral batches using p24 antigen ELISA (PerkinElmer Life Sciences, Waltham, MA, USA).
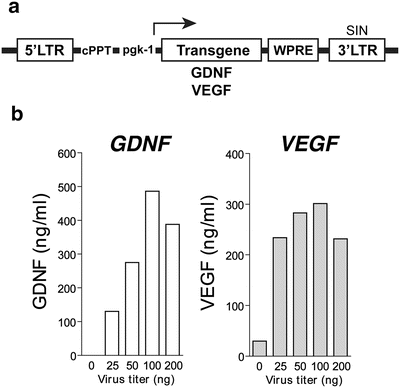

Fig. 2
hMSCs can be genetically engineered by lentiviral infection to stably express growth factor s. (a) Schematic of lentiviral construct. LTR long terminal repeat, pgk-1 mouse phosphoglycerate kinase 1 promotor, WPRE post transcriptional regulatory element of woodchuck hepatitis virus , cPPT central polypurine tract, SIN self-inactivating. (b) After infection with serial dilutions of viruses, ELISA can detect GDNF and VEGF proteins in the conditioned medium [10, 11]
2.3 hMSC Transplantation in the Rat Muscle
1.
2.
Transplant medium: DMEM high glucose (GlutaMAX™; Life technologies, Carlsbad, CA, USA), 0.5 % BSA (Sigma-Aldrich, St. Louis, MO, USA), filtered (see Note 6 ).
4.
Cyclosporine solution: Mix 3 ml of SandImmune (100 mg/ml; Novartis, Basel, Switzerland) and 27 ml of extra virgin olive oil. Final concentration is 10 mg/ml. Filter the mixture using a 0.45 μm bottle top filter. Transfer it in a glass vial, cap with rubber stopper, and cover the bottle with aluminum foil to shield light (see Note 8 ).
5.
1 ml disposable syringes.
6.
A 100 μl Hamilton syringe (Reno, NV, USA).
7.
30- or 33-gauge needles.
3 Methods
Primary human tissue, hMSC cultures, and any medium removed from these cultures are hazardous waste and should be contained and discarded in appropriate biohazard containers.
All culture procedures should be completed in a laminar-flow culture hood unless indicated otherwise. Cultures should be maintained in a humidified incubator at 37 °C and 5 % CO2. Media should be warmed in a 37 °C water bath prior to use.
All animal procedures must be carried out in accordance with the guidelines for an Institutional Animal Care and Use Committee (IACUC) and must conform to National Institutes of Health standards of animal care and use.
3.1 Preparation of hMSC Culture
3.1.1 Thawing of hMSCs
1.
Thaw the cryovial quickly in a 37 °C water bath.
2.
Sterilize the cryovial with isopropanal.
3.
Transfer contents to a 15-ml conical tube and slowly add 10 ml of the hMSC culture medium.
4.
Centrifuge at 168 × g for 5 min at room temperature.
5.
Remove the supernatant, re-suspend in 10 ml of the hMSC culture medium, and plate in a T25 culture flask at approximately 30–40 % confluence.
6.
Check the cells daily using a microscope. Passage cells at approximately 80–90 % confluency.
3.1.2 Passaging of hMSCs
1.
Remove supernatant from the flask.
2.
Rinse once with 5 ml of DPBS. Add the PBS gently to not disturb the cell monolayer.
3.
Add 3 ml of 0.05 % trypsin-EDTA and incubate in a CO2 incubator for 3–4 min.
4.
Tap the flask vigorously and check under the microscope. Make sure that all cells are properly detached.
5.
Add 3 ml of pre-warmed hMSC culture medium.
6.
Pipette up and down, rinsing the bottom of the flask. Make sure that all cells are collected and do not remain adhered to the flask.
7.
Transfer the cells to a 15 ml conical tube.
8.
Centrifuge at 168 × g for 5 min at room temperature.
9.
Remove the supernatant, re-suspend in 15 ml of the hMSC culture medium, and plate in a new T75 culture flask.
10.
Passage hMSC lines approximately once per week (see Note 9 ). If starting from a 90 % confluent T75 flask passage in 1:3 ratio, prepare 3T75 flasks with 12 ml of hMSC culture medium each. Re-suspend the cells in 9 ml of the hMSC culture medium and add 3 ml of the cell suspension to each T75.
3.2 Lentiviral Infection of hMSCs
Lentiviral infection can be used to stably integrate a gene of interest. Our previous work has shown that both rat and human neural progenitor cells can be efficiently infected with lentiviral constructs [15, 16]. We used this experience with neural progenitor cells to prepare hMSC expressing GDNF and/or VEGF [10, 11]. The same viral construct has been used for constitutive expression of growth factor s under the control of the mouse pgk-1 promoter (see Note 10 ).
1.
Prepare a single cell suspension of hMSCs in hMSC culture medium as described above (see Subheading 3.1).
2.
Count cells in a 10 μl aliquot using a hemocytometer and assess viability using 0.4 % trypan blue (see Note 11 ).
3.
Resuspend the cells in hMSC culture medium as 25 cells/μl in a 15 or 50 ml conical tube (depending on the number of the wells to be used).
4.
Add 400 μl of the cell suspension in a 24-well plate and incubate in a CO2 incubator.
5.
After 24 h, remove the medium and add 300 μl of the culture medium containing various virus titers (0–200 ng/p24/per well) to each well (see Note 12 ).
6.




After incubating for 24–72 h in a CO2 incubator, collect the conditioned medium and store it in a −20 °C freezer. Rinse cells once with 300 μl DPBS and then add 300 μl per well of 0.05 % trypsin-EDTA. Split the cells as described above (see Subheading 3.1) and scale up the culture as necessary for further studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






