Felbamate
Edward Faught
HISTORICAL BACKGROUND
When felbamate (FBM) was introduced to clinical practice in the United States in July 1993, there had been no major new antiepileptic drugs (AEDs) for 15 years, and enthusiasm ran high. Dangerous side effects were not anticipated based on the experience of 2100 patients enrolled in clinical trials, but in 1994, FBM was found to be associated with a high incidence of aplastic anemia (1). Compared with the 120,000 individuals who were treated with the agent in the first year, only 14,000 to 15,000 patients worldwide are now receiving FBM therapy (2). FBM remains available for patients with refractory seizures who respond poorly to other medications, with the number of treated patients remaining stable in recent years (2).
FBM is one of a series of dicarbamate compounds, including the minor tranquilizer meprobamate, synthesized in the 1950s. With no tranquilizing or sedative effects, FBM was not immediately useful. In 1986, efficacy in a wide range of experimental seizure models, with relatively low neurotoxicity, was reported (3).
Encouraging results from pharmacokinetic and pilot toxicity studies were described (4,5). Two controlled clinical trials (6,7) in patients with refractory partial seizures suggested modest efficacy of FBM as an adjunctive agent. Significant pharmacokinetic drug interactions, in part, prompted testing of FBM as a monotherapy agent. The FBM development program was characterized by the use of two innovative monotherapy clinical trial designs, which have since been applied to test several new AEDs.
CHEMISTRY AND MECHANISM OF ACTION
Chemistry
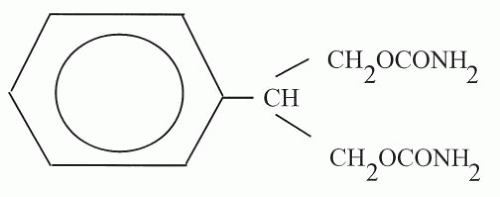
FBM (2 phenyl-1,3-propanediol dicarbamate) differs from meprobamate by having a phenyl group, rather than an aliphatic chain, at the 2-carbon position (Fig. 65.1). FBM is lipophilic and relatively insoluble in water. No parenteral preparation for humans is available, but intravenous administration in mice has been achieved by encapsulating FBM molecules with hydrophobic diketopiperazine microspheres (8). The molecular weight of the agent is 238.24 (9).
Antiepileptic Profile in Animals
FBM displayed high protective indices (toxic dose50/effective dose50) against both the tonic phase of maximal electroshock seizures and subcutaneous pentylenetetrazol-induced seizures in rodents (3). The agent increases seizure threshold and reduces seizure severity in fully amygdala-kindled rats (10), but it is not known whether it inhibits kindling development.
Mechanisms of Action
Evidence points to four mechanisms of antiepileptic action, all antiexcitatory. FBM interferes with voltage-gated sodium channels, resulting in blockade of sustained repetitive neuronal firing and prevention of seizure spread (11). Although earlier data suggested that FBM interfered with the function of N-methyl-D-aspartate (NMDA)-type glutamate receptors by competitive inhibition of glycine, an obligatory coagonist (12), recent studies indicate that it is non-competitive with respect to both glycine and glutamate (13). FBM has been found to bind selectively to some NMDA-receptor subtypes, especially those containing NR2B subunits (13,14). In either case, the net effect is reduction of NMDA-receptor-modulated cationic conductance. Subtype specificity may account for the lack of side effects with FBM that are typical of such NMDA receptor blockers as MK-801 (15). The third proposed mechanism of action involves non-NMDA-excitatory amino acid receptors. Not only does FBM protect against seizures induced by quisqualate and kainate (11), but also against seizures induced by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), (16). Glutamate
the natural agonist for this receptor subtype. Finally, the agent inhibits voltage-activated calcium currents at clinically relevant concentrations (17). This may inhibit release of excitatory neurotransmitters and underlie the anti-absence activity of FBM.
the natural agonist for this receptor subtype. Finally, the agent inhibits voltage-activated calcium currents at clinically relevant concentrations (17). This may inhibit release of excitatory neurotransmitters and underlie the anti-absence activity of FBM.
One or more of these antiexcitatory mechanisms may be responsible for a neuroprotective effect of FBM, which reduced neuronal damage in a rat model of hypoxia-ischemia (18), protected CA1 hippocampal neurons from apoptosis in a gerbil ischemia model (19), and exhibited neuroprotective effects in a rat model of status epilepticus (20).
FBM and related compounds have potential as treatments for status epilepticus. Fluorofelbamate, a new FBM analog, very effectively terminates seizures in the rat self-sustaining status epilepticus (SSSE) model (21). A major therapeutic problem is the relative inefficacy of phenytoin, phenobarbital, and benzodiazepines in late-stage status epilepticus. In the SSSE model, fluorofelbamate was effective but phenytoin and phenobarbital were not, even after 40 to 70 minutes of continuous seizures. Furthermore, unlike the other agents, FBM attenuated the development of recurrent, spontaneous seizures after SSSE, suggesting that it is antiepileptogenic (21).
ABSORPTION, DISTRIBUTION, AND METABOLISM
FBM is well absorbed; more than 90% of 14C-labeled FBM, or its metabolites is recovered in urine and feces after oral administration (22), and the rate and extent of absorption are not affected by food or antacids (23).
FBM readily crosses the blood-brain barrier, with brain and cerebrospinal fluid concentrations in animals close to plasma concentrations (9). In human plasma, only 22% to 25% of FBM is reversibly bound to proteins (primarily albumin), independent of dosage (24), and protein-binding interactions are thus insignificant.
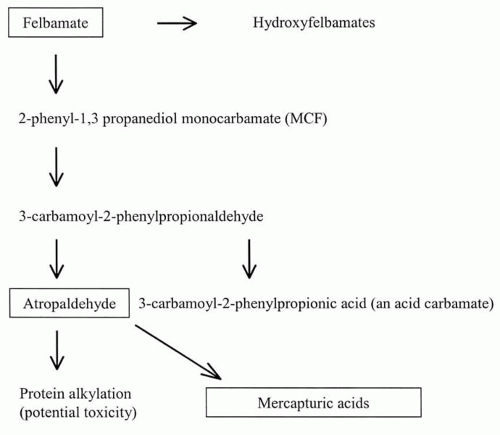
Of the absorbed FBM dose, 30% to 50% is excreted in the urine unchanged (22,25), with renal clearance decreasing to 9% to 22% of the total dose in patients with renal dysfunction (25). The remainder is metabolized by the liver by several pathways (26). (Fig. 65.2) Pharmacokinetic differences in patients 67 to 78 years of age were minor (27).
FBM exhibits linear first-order pharmacokinetics over a dose range of 1200 to 6000 mg per day in humans (28). Peak plasma concentration is reached 3 hours after administration of an oral dose, and concentrations increase proportionally after single and multiple doses (26). Monotherapy with FBM 3600 mg per day produced a mean trough plasma level of 78.4 μg/mL (range, 23.7 to 136.6 μg/mL) in one study (29) and a mean (± standard deviation) level of 65 (± 23) μg/mL after 112 days in another (30).
The terminal elimination half-life of 20 hours (range, 13 to 23 hours) with FBM monotherapy decreases to 13 to 14 hours in the presence of phenytoin or carbamazepine (5). The apparent volume of distribution is 0.8 L/kg (31).
Steady-state plasma levels are achieved approximately 4 days following initiation of therapy (26).
Steady-state plasma levels are achieved approximately 4 days following initiation of therapy (26).
EFFICACY
FBM is approved for use in the United States as either adjunctive therapy or monotherapy for patients older than 14 years of age with partial seizures, with or without generalization, and as adjunctive therapy for patients of any age with Lennox-Gastaut syndrome and its component seizure types (31).
Partial-Onset Seizures
The initial clinical trials of FBM as an adjunct to carbamazepine (6,7) or phenytoin (6) therapy used standard designs and yielded unimpressive results. Reductions in seizure frequency were either small (6) or not significantly different (7) from those with placebo. In 1998, new designs for AED trials were proposed in a workshop sponsored by the Epilepsy Branch of the National Institutes of Health (32). Clinical investigators of FBM were the first to use these designs.
In a presurgical evaluation trial (33), FBM or placebo was added to the AEDs in use at the conclusion of electroencephalogram (EEG)-video monitoring. The primary endpoint was time to occurrence of the fourth seizure or 29 days, whichever came first. Of patients randomized to placebo, 88% experienced a fourth seizure before day 29, compared with 46% taking FBM (p = 0.03). In outpatient monotherapy trials (29,30), standard therapy was withdrawn over 28 days, and FBM 3600 mg per day or valproate 15 mg/kg per day was substituted. The valproate dose represented a compromise between a placebo control, which might have been unsafe, and a full-dose active control, which might have called the conclusions into question (34). The end point was “escape” from treatment, defined as treatment failure according to predetermined criteria, including a doubling of seizure frequency during any 2 days or 1 month, compared with pretreatment baseline. Patients taking low-dose valproate met escape criteria more often than did FBM-treated patients (86% versus 14%, respectively, of 42 patients in the single-center study [29]; 78% versus 40%, respectively, of 95 patients in the multicenter study [30]). The “presurgical” design was also repeated as a monotherapy trial and further confirmed monotherapy efficacy (35).
Open-label experience with adjunctive FBM-therapy has been more encouraging than the original adjunctive-use controlled trials. In one open-label assessment, 20% of 111 adults had greater than 50% reductions in seizure frequency (36). Adjunctive open-label use reduced seizure frequency by 53% among a group of 30 children aged 2 to 17 years (37). No direct comparisons of FBM efficacy with that of other AEDs are available.
Lennox-Gastaut Syndrome
FBM 45 mg/kg per day was used as adjunctive therapy, usually along with valproate, in a multicenter, double-blind, controlled trial of 73 patients (38). Atonic seizures (drop attacks) were reduced by 34% and all seizures by 19%, versus a 9% decrease and a 4% increase, respectively, in these parameters with placebo. A difference in atypical absence frequency was not proved. Parents also reported increased alertness and verbal responsiveness in their children. During a 12-month, open-label follow-up, seizure frequency decreased by 50% in FBM-treated patients, compared with 15% in the placebo group (39,40). FBM may have a synergistic effect with valproate: In a study of 13 patients with Lennox-Gastaut syndrome, drop attacks were reduced by 40% and total seizures by 50% when FBM was added—effects not completely explained by the observed 12.7% increase in valproate levels (41).
Other Seizure Types
Secondarily generalized tonic-clonic seizures respond to FBM treatment (30,42). Reduction in the number of generalized tonic seizures as part of the Lennox-Gastaut syndrome has been demonstrated (38). There are reports of efficacy in small, uncontrolled series of patients with infantile spasms (43), primary generalized seizures (42,44), absence seizures (45), atypical absence seizures not part of the Lennox-Gastaut syndrome (46), and juvenile myoclonic epilepsy (47).
DRUG INTERACTIONS
Clinically significant interactions with phenytoin, carbamazepine, valproate, and phenobarbital have been established (Table 65.1). FBM is metabolized by the hepatic cytochrome P450 (CYP) system, primarily by CYP2E1 and to a minor extent by CYP3A4 (48). Thus, CYP2E1 inhibitors such as chlorzoxazone increase FBM levels, whereas CYP3A4 inhibitors such as erythromycin have little effect (49). Drugs such as carbamazepine, phenytoin, and phenobarbital, which induce CYP3A4, increase FBM clearance (48). FBM inhibits phenytoin and phenobarbital clearance by inhibiting CYP2C19 (48).
Effect on Carbamazepine
When FBM is added to carbamazepine, levels of carbamazepine decrease 20% to 30%, but carbamazepine epoxide (CBZ-E) levels increase by 50% to 60% (7,50,51). FBM induces CYP3A4-mediated carbamazepine metabolism and inhibits epoxide hydrolase, which metabolizes CBZ-E (52). These effects can cause clinical toxicity related to the elevated CBZ-E level. The carbamazepine-FBM combination
is often associated with headache—a probable pharmacodynamic interaction.
is often associated with headache—a probable pharmacodynamic interaction.
TABLE 65.1 INTERACTIONS OF FELBAMATE AND OTHER AEDS | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree