Chapter 124 Fibromyalgia and Chronic Fatigue Syndromes
Abstract
Other chapters in this volume address the diagnostic features and management of those sleep disorders in which tiredness is a synonym for sleepiness; furthermore, the evaluation of the medical and psychiatric conditions that result in tiredness are outside the scope of this chapter. This chapter will focus on the assessment and management of those chronically tired patients who complain of unrefreshing sleep or chronic mental and physical fatigue in the absence of physical or laboratory evidence for a primary medical disease or behavioral evidence for a primary psychiatric disorder. Often, when generalized and ill-defined musculoskeletal pain and tenderness in specific anatomic regions accompany the fatigue, these people acquire descriptive labels such as chronic fatigue syndrome1 or fibromyalgia syndrome.2
Epidemiology
Epidemiologic studies reveal that 23% of the adult population in the United States report having experienced the symptom of persistent fatigue sometime during their life. A cross-national epidemiologic study of primary care physicians shows that unexplained substantial fatigue occurring for more than 1 month affects approximately 8% of patients, with a range from between 12% and 15% in Berlin, Santiago, and Manchester to a low prevalence of between 2% and 4% in Ibadan, Verona, Shanghai, Seattle, and Bangalore. Within these variable prevalence clusters of patients with unexplained fatigue lie smaller groups of overlapping primary diagnoses, which include CFS and FMS. In the United States and Japan, between 0.4% and 1.5% of people are affected with CFS (i.e., these patients complain of persistent fatigue for more than 6 months).3,4 FMS affects approximately 2% of the population (more than 80% of them women), and debilitating fatigue affects between 76% and 81% of these patients.2 Because of the similarities of the symptoms, there is considerable overlap between the diagnoses of CFS and FMS, which are also comorbid with such regional pain syndromes as temporomandibular joint disorder, irritable bowel syndrome, migraine, and tension headaches.5
Pathogenesis
More than 30 years ago, the experimental disruption of slow-wave sleep (SWS) with noise in healthy sleeping subjects induced both musculoskeletal pain and fatigue symptoms.1 Subsequently, a variety of studies have confirmed the fundamental observation of the artificial induction of pain and fatigue symptoms by disruption of SWS.6 However various procedural differences may have resulted in some differences among the studies. For example, one study that did not employ an adaptation night failed to show significant differences in tender point threshold after noise-induced sleep disruption. Another study showed altered pain thresholds, but there was no associated increase in alpha sleep activity by electroencephalography (EEG).6,7 It is uncertain whether computer-generated frequency analysis used in this study might have excluded noise-induced EEG arousals from sleep.
Experimental total sleep deprivation or specific deprivation of SWS or rapid eye movement (REM) sleep affects pain measures in normal subjects. For example, 40 hours of total sleep deprivation reduces pain thresholds, which return to baseline values specifically after SWS recovery.3 Studies of the effects of either REM or SWS deprivation followed by a night of recovery show that both conditions reduce pressure-pain tolerance thresholds. An increase in SWS on the recovery night is associated with an increase in the pain tolerance threshold. This research showed that sleep deprivation has a specific effect on inducing a hyperalgesic state but does not alter somatosensory functions. The fragmentation of normal human SWS is shown to interfere with the nervous system’s natural inhibitory response to painful noxious stimuli as well as causing increased sensitivity to various nonpainful stimuli (e.g., bright light, loud sounds, strong odors). These symptoms of hypersensitivity to various perceived noxious stimuli typically occur in patients with FMS.
There are numerous reports of disordered EEG sleep in patients with FMS. The studies show protracted sleep-onset latencies, a reduced sleep efficiency index, a reduction of SWS and REM sleep, increased motor activity during sleep, generalized restlessness,8 and an increase in alpha EEG activity during non-REM sleep. The alpha EEG non-REM sleep anomaly is the most extensively studied correlate of nonrestorative sleep, especially as it applies to CFS and FMS. However, it is not specific to these conditions, and some studies have reported a low sensitivity.3,4,9
Patients with FMS are often found to have fragmented sleep as a result of sleep-related periodic arousal disturbances that occur over the course of the night. These periodic sleep-related disturbances include involuntary periodic limb movements (PLM) or restless legs, sleep apnea, and an underlying periodic arousal disturbance in the sleep EEG. This periodic EEG sleep disorder was initially described as a high rate of periodic K-alpha, and it is now better known as the cyclic alternating pattern (CAP).6 The presence of a high frequency of CAP in EEG sleep, which occurs in sequences of 20 to 40 seconds, may interfere with the typical EEG K-spindle complex that typically occurs in stage 2 non-REM sleep. This sleep finding in FMS may account for the finding of a low frequency of sleep spindles. The high index of periodic arousal disturbance in the sleep EEG is an indicator of sleep instability. It is accompanied by less efficient and unrefreshing sleep and is correlated to the severity of clinical symptoms in patients with FMS.
Despite the absence of specific structural pathology, there is evidence for disordered metabolic functions that involve the sleeping/waking brain. These include elevation of cerebral spinal fluid substance P, decrease in growth hormone and its metabolites, and disturbances in the hypothalamic-cortical-adrenal axis.10–15 Furthermore, specific dysfunctions in neurotransmitter functions have been shown to contribute to bodily sensitivity and disordered sleep. For example, inhibition of serotonin (5-HT) synthesis by p-chlorophenylalanine induces insomnia and a hyperalgesic state in animals and humans. The increased levels of substance P that are found in the cerebrospinal fluid of patients with FMS led to experimental studies that demonstrated that substance P operates through a neurokinin (NK) pathway to influence nociception and sleep. Intracerebral ventricular administration of substance P in sufficient quantities that did not induce nociceptive response in mice delayed onset of sleep and provoked awakenings from sleep. An NK-1 receptor antagonist reversed the interfering effect on sleep by substance P. This research demonstrates that the blocking of the substance P–induced insomnia by prior treatment of the mice with NK-1 receptor antagonist provides support to the notion of the arousing effect of substance P on the sleeping/waking brain. Moreover, the research provides an experimental animal model for studying sleep disturbances and musculoskeletal pain in FMS.
Some studies in FMS with functional neuroimaging support the hypothesis of central pain augmentation. The most recent study showed that patients with FMS have a decrease in gray-matter volume in the prefrontal cortex, the amygdala, and the anterior cingulate cortex. The duration of pain or functional pain disability did not correlate with gray matter volumes. One possible conclusion is that volume reductions might be a precondition for central sensitization in fibromyalgia.15 Alterations in dopamine metabolism might contribute to the associated changes in gray matter volume and density.16
Increased overnight sympathetic activity as measured electrocardiographically is consistent with the notion of circadian autonomic metabolic dysfunction associated with an arousal disturbance during the sleep of patients with fibromyalgia.17 In addition to the chronobiological physiologic abnormalities, there are diurnal changes in behavior. FMS subjects with light unrefreshing sleep have diurnal impairment in speed of performance on complex cognitive tasks, which are accompanied by sleepiness, fatigue, and negative mood. Such psychological impairment could account for the functional disabilities that are encountered in a work environment and in social behavior.
Clinical Features
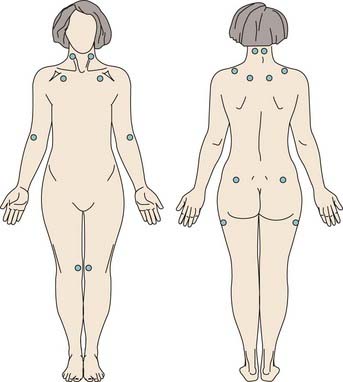
The clinical criteria for CFS are shown in Box 124-1, and those for FMS are shown in Box 124-2. The American College of Rheumatology criteria emphasize pain and tenderness (Fig. 124-1) for the diagnosis of FMS, but this may actually limit sensitivity and specificity. An evaluation for FMS should investigate complaints of chronic fatigue, unrefreshing sleep, cognitive difficulties, and psychological distress. Whereas fatigue, postexertional malaise, and impairment in cognitive functioning are the focus of attention in patients with CFS, unrefreshing sleep, chronic muscular pain, and tenderness also prevail. These symptoms may follow a viral illness or a physical or psychological traumatic event (e.g., an automobile whiplash injury or a work-related soft tissue injury), or they may appear for no apparent reason. FMS may occur in the context of potentially disabling arthritic disease (e.g., rheumatoid arthritis) or connective tissue disease (e.g., systemic lupus erythematosus), human immunodeficiency virus infection, or Lyme disease.
Box 124-1
From Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994;121:953-959.
Criteria for Chronic Fatigue Syndrome (Centers for Disease Control, 1994)
In patients with either CFS or FMS, the quality of sleep is usually impaired. More than 80% of patients with chronic fatigue describe significant impairment in their sleep quality.18 Patients commonly complain of light or superficial sleep, describing sensitivity to noise or inability to turn off thinking during sleep, so their sleep is unrefreshing. Their mental fatigue results in word lapses, word confusions, and problems in multitasking, so they are slower in performing such tasks. However, they do not make more errors than unaffected individuals.19 Their sensory sensitivities may include lowered tolerance to noise, noxious smells or strong perfumes, and bright light. In addition to external sensory hypersensitivities, they may describe internal sensitivities (e.g., intolerance to certain foods and symptoms of irritable bowel syndrome or irritable bladder).
Diagnosis
Clinical Assessment
Specific enquiry about the quality of sleep (e.g., whether sleep is light and unrefreshing) is essential. Sleep–wake habits can be assessed with the aid of a sleep diary. Attention should be directed to potential agents that could interfere with sleep—for example, excessive use of caffeine, alcohol, nicotine, and certain classes of drugs. Some patients may have loud snoring and interruptions of breathing, which could indicate sleep apnea. Others may describe dysesthesia and uncontrollable leg movements in the evening and restlessness or kicking during sleep, which characterize restless legs syndrome and involuntary PLM disorder.20,21 Tiredness as the result of excessive daytime sleepiness should be differentiated from physical and central fatigue. Routine laboratory tests help to exclude a primary medical disease. The routine use of expensive tests (e.g., imaging studies) is not required. However, patients with FMS or CFS may have a concomitant rheumatic or connective tissue disease that requires careful medical diagnostic appraisal. Furthermore, because unrefreshing sleep, fatigue, and cognitive impairment are often accompanied by anxiety or depression, assessment of psychological disturbances is required as part of an overall plan of management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree