Like oroalimentary automatisms, the hand automatisms suggest onset from the mesial temporal region. In extratemporal seizures with unilateral tonic posturing, thrashing to-and-fro movements, which are more proximal and not discrete, are sometimes seen in the opposite limb.
Eye blinking or fluttering may be observed. Although usually symmetric, unilateral blinking has been reported ipsilateral to the seizure focus (63). A mechanism similar to unilateral hand automatisms may be operative, but this has not been documented. Rapid, forced eye blinking when the seizure begins is thought to indicate occipital lobe onset (63). Seizures arising from the occipital region may produce version of the eyes to the opposite side (54).
Truncal or body movements may be seen, usually in the middle or late third of the seizure, when the patient attempts to sit up, turn over, or get out of bed (34).
Bicycling or pedaling movements of the legs are more commonly observed in focal seizures with impaired consciousness arising from the mesial frontal and orbitofrontal regions (30) than in temporal lobe seizures. They are sometimes seen in temporal lobe seizures but probably reflect spread of the ictal discharge to the mesial frontal cortex.
Mimetic automatisms, with changes in facial expression, grimacing, smiling, or pouting, are common in focal seizures with impaired consciousness (54). Crying has been noted in focal seizures with impaired consciousness arising from the nondominant temporal lobe (54).
Sexual or genital automatisms such as pelvic or truncal thrusting, masturbatory activity, or grabbing or fondling of the genitals are relatively uncommon during focal seizures with impaired consciousness. However, they have been reported in focal seizures with impaired consciousness of frontal lobe origin, as well as in those arising from the temporal lobes (54). Leutmezer et al. (64) postulate that discrete genital automatisms such as fondling or grabbing the genitals are seen in temporal lobe seizures, whereas hypermotoric sexual automatisms such as pelvic or truncal thrusting usually occur in frontal lobe seizures.
So, in summary, although various types of automatisms may have a useful localizing value, it is mainly unilateral distal limb automatisms with contralateral dystonia that is useful as a lateralizing sign.
Postictal Nose Wiping
Nose wiping or rubbing that occurs within 60 seconds of the seizure end has good localizing and lateralizing value: it occurs in 50% to 85% of temporal lobe epilepsy patients but in only 10% to 33% of extratemporal epilepsy patients. It is performed with the hand ipsilateral to the epileptogenic focus in 75% to 90% of the cases when seen in the context of a temporal lobe automotor seizure but has no lateralizing value when seen with an extratemporal seizure. Postulated mechanisms leading to its occurrence include ictal activation of the amygdala with subsequent olfactory hallucinations, or increased nasal secretions, and postictal contralateral hand movement abnormalities or neglect (54,65).
ELECTROENCEPHALOGRAPHIC FINDINGS
Interictal Electroencephalography
Most focal seizures with impaired consciousness and automatisms arise from the anterior temporal regions of one side or the other. Temporal intermittent rhythmic delta activity is found in up to 28% of patients evaluated for temporal lobe epilepsy as opposed to only 0.3% of the general population and is therefore felt to be significantly predictive of temporal lobe epilepsy. Bitemporal sharp-wave foci are noted in 25% to 33% of patients and may be independent or synchronous. Mesial temporal spikes may not be well seen at the surface, and intermittent rhythmic slowing may be the only clue to deep-seated spikes (66). On a single routine EEG recording, 30% to 40% of patients may have normal interictal findings; activating techniques can reduce this to approximately 10% (67).
At the scalp, the field of mesial temporal spikes is often maximal at the anterior temporal electrodes (T1 or T2, FT9 or FT10). When nasopharyngeal or sphenoidal electrodes are used (especially in prolonged monitoring), the amplitude of the spike is usually maximal at these electrodes, consistent with their origin in the amygdalar–hippocampal region (Fig. 12.1).
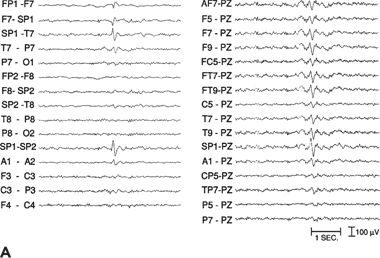
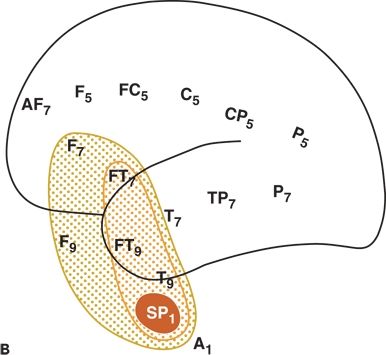
Figure 12.1. Left mesial temporal lobe. Interictal sharp-wave focus (left). A and B:Distribution of the field of an interictal spike from a patient with temporal lobe epilepsy. The spike amplitude is maximal at SP1 (measured on referential recordings); >90% at T9, FT9, and FT7 electrodes; and >70% at F7 and F9.
Less frequently, sharp-wave foci are seen in the midtemporal or posterior temporal region. Interictal foci may be mapped according to amplitude, and the relative frequency of various sharp-wave foci may be taken into account during monitoring of epilepsy surgery candidates. A fair degree of correlation is present between the predominant spike focus and side of ictal onset [63% in Wieser et al. (68) series of 133 patients]. Hyperventilation may activate focal temporal slowing or spikes and may provoke a clinical seizure.
In 10% to 30% of patients with focal seizures with impaired consciousness, an extratemporal focus is seen, usually in the frontal lobes (Fig. 12.2). In some patients with mesial frontal foci, the interictal discharge may take the form of a bifrontal spike-and-wave discharge.
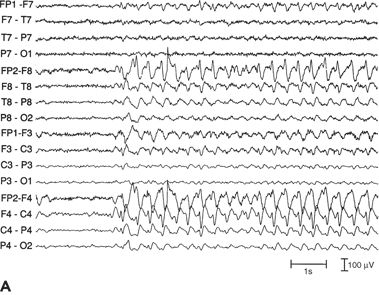
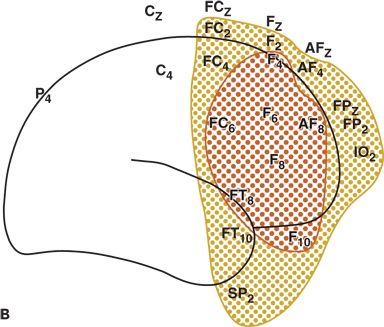
Figure 12.2. Run of focal spike-and-wave discharge in the right frontal lobe with no clinical signs (left) and right frontal lobe, interictal spikes (right). A and B:Distribution of the field of interictal spikes occurring in runs from a patient with frontal lobe complex partial seizures. The field is widespread, involving most of the right frontal convexity.
Care should be taken to exclude nonepileptiform sharp transients such as benign epileptiform transients of sleep or small sharp spikes, wicket spikes, and 14- and 6-Hz spikes. Evidence suggests that when benign epileptiform transients of sleep occur in epileptic individuals, they do so frequently and in runs (69). Transients resembling benign epileptiform transients of sleep sometimes are found to be maximal at the sphenoidal electrode; such discharges should be interpreted cautiously.
Ictal Electroencephalography
Although interictal EEGs may show normal findings in some patients with focal seizures with impaired consciousness, ictal changes are seen in 95% of patients (except during isolated auras) (70). In frontal lobe seizures from the mesial frontal or orbitofrontal cortex, ictal and interictal activities may not be reflected at the surface or are often masked by electromyographic and movement artifacts (Fig. 12.3).
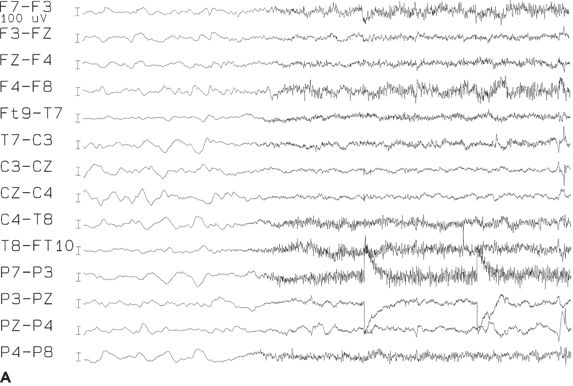
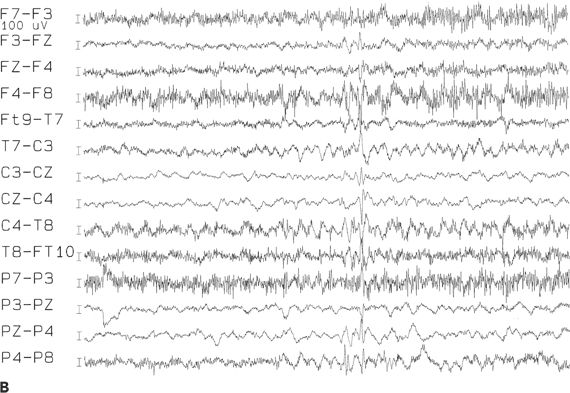
Figure 12.3. A and B: Ictal onset of a frontal lobe complex partial seizure arising out of sleep, beginning with low-amplitude fast rhythms, followed by rhythmic slowing near the vertex. The electroencephalographic seizure pattern cannot be lateralized.
An electrodecremental pattern is seen at the onset of a focal seizure with impaired consciousness in about two-thirds of patients. It is usually quite diffuse (perhaps owing to an associated arousal); if focal or accompanied by low-voltage fast activity, it has lateralizing significance. The low-voltage fast activity, best seen with depth electrodes, may appear only as flattening at the surface. Alternatively, diffuse bitemporal slowing, higher on one side, may occur (70,71).
Approximately 50% to 70% of patients with temporal lobe epilepsy exhibit a so-called prototype pattern (71) consisting of a 5- to 7-Hz rhythmic θ discharge in the temporal regions, maximum at the sphenoidal electrode (Fig. 12.4). This pattern may appear as the first visible EEG change or follow diffuse or lateralized slowing in the δ range (often within 30 seconds of clinical onset). Depth electrode studies have shown this pattern to have 80% accuracy in localizing the onset to the ipsilateral mesial temporal structures (72). Postictal slowing is also helpful in lateralization. In patients with unitemporal interictal spikes, the lateralizing value of the ictal data was excellent (70). Seizure rhythms at ictal onset confined to the sphenoidal electrode are often seen in patients with mesial temporal lobe epilepsy as opposed to those with non–temporal lobe epilepsy. Use of coronal transverse montages incorporating the sphenoidal electrodes may permit earlier identification of seizure onset (70).
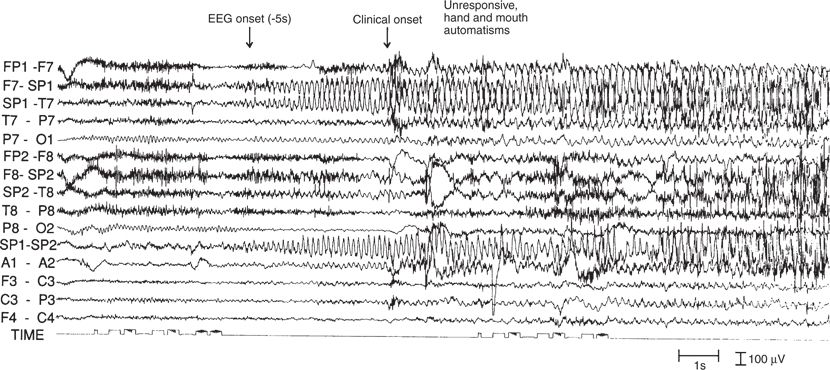
Figure 12.4. Ictal onset of a complex partial seizure (same patient as in Fig. 12.1). A brief electrodecremental response in the left temporal region is followed by the buildup of a rhythmic 5- and 6-Hz theta pattern, maximal at the left sphenoidal electrode. The electroencephalographic changes preceded the clinical onset by 5 seconds.
Although previous reports described false lateralization on the basis of scalp EEG, subsequent systematic studies have shown this to be infrequent except in the presence of a structural lesion that may mask or attenuate the amplitude of the ictal discharge on that side (70). Although lateralization from scalp EEG is usually satisfactory, localization within a lobe is sometimes incorrect, because seizures from an extratemporal site may spread to the temporal lobe and produce similar EEG patterns. The ictal discharge may then propagate to the rest of the hemisphere, or it may propagate bilaterally. Spread to the opposite temporal lobe is common. With some frontal lobe seizures, scalp ictal changes are difficult to appreciate because of electromyographic and movement artifacts. Occasionally, a generalized spike-and-wave discharge with a mesial frontal focus is seen (70).
PATHOPHYSIOLOGY OF IMPAIRED CONSCIOUSNESS IN FOCAL SEIZURES
Gloor believed in 1986 that while a “satisfactory explanation of consciousness . . . may never be possible . . . there are, however, aspects of conscious experience such as perception, cognition, memory, affect, and voluntary motility that are open to neurobiologic research” (4). Since that time, and because of multiple “neurobiologic research” attempts, significant advances have been made in the understanding of altered consciousness in the setting of focal seizures, and various mechanisms have been proposed.
Epileptic Activation of Subcortical Structures, Mainly the Thalamus and Upper Brainstem
Close connections exist between the prefrontal cortex and the nonspecific thalamic nucleus and the midline region of the intralaminar thalamic complex. Since epileptiform discharges arising from various regions within the frontal lobe—including the intermediate frontal region, orbitofrontal region, and cingulate gyrus—may elicit dialeptic seizures, and generalized discharges can be seen with epileptic activation of the mesial frontal lobe (secondary bilateral synchrony), it has been proposed that rapid epileptic spread from all of those frontal regions to the reticular formation is actually responsible for the impaired consciousness observed in frontal lobe epilepsy (1).
In temporal lobe epilepsy, a similar mechanism with additional spread to the upper brainstem structures has been suggested based on ictal SPECT perfusion studies (73) and supported by intracranial recordings demonstrating the more prominent occurrence of bilateral frontoparietal slow waves in seizures with impaired consciousness as opposed to “simple partial” seizures (74). The complete loss of consciousness seen in generalized seizures has been attributed to the involvement of both cortical and subcortical structures with epileptic activity (75). As such, the bilateral involvement of the thalamus and upper brainstem occurring early in generalized epilepsy or as a late spread phenomenon from the mesial temporal structures can selectively impair the frontoparietal association cortices and default mode networks, leading to loss of awareness (76).
Epileptic Activation of the Limbic System
Invasive EEG recordings have shown that ictal discharges in the mesial temporal lobes may be associated with the dialeptic symptomatology (1). In a recent study of 134 patients with focal seizures with impaired consciousness, Heo et al. found that interictal epileptiform discharges (IEDs) localized primarily to the temporal region and were more frequently detected in patients who were unaware of their seizures (94%) than in those who were aware (55%). Bilateral independent IEDs were found more frequently in the unawareness group than in the awareness group (48% vs. 13%). The bilateral presence of lesions was also more frequent in the unawareness group than in the awareness group (16.1% vs. 4.9%). The authors concluded that complete loss of consciousness was caused by rapid spread of ictal discharges to the contralateral hemisphere in association with bilateral independent IEDs and bilateral presence of lesions (77).
Epileptic Disturbance of the Normal Balance Between Excitation and Inhibition of Various Cortical/Subcortical Networks
Some authors suggest that arrest of activity during a seizure may be the result of either interference with the normal activity of the primary motor cortex or epileptic activation of the negative motor areas during frontal lobe involvement, or both (1). Abnormal increased activity in frontoparietal association cortex and related subcortical structures is associated with loss of consciousness in generalized seizures. Abnormal decreased activity in these same networks may cause loss of consciousness in focal seizures with impaired consciousness. Thus, abnormally increased or decreased activity in the same networks can cause loss of consciousness. Information flow during normal conscious processing may require a dynamic balance between these two extremes of excitation and inhibition (78,79).
References
1. Noachtar S. Dialeptic seizures: localizing and lateralizing value. In: Lüders HO, ed. Textbook of Epilepsy Surgery. United Kingdom: Informa; 2008:479–487.
2. Ebner A, Dinner DS, Noachtar S, et al. Automatisms with preserved responsiveness: a lateralizing sign in psychomotor seizures. Neurology. 1995;45(1):61–64.
3. Serrano-Castro PJ, Alonso-Morillejo E, Pozo-Munoz C, et al. Characteristics of temporal lobe epilepsy with no ictal impairment of consciousness. Clin Neurol Neurosurg. 2013;115(8):1338–1342.
4. Gloor P. Consciousness as a neurological concept in epileptology: a critical review
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








