INTRODUCTION
The last few years have seen a rapid upsurge in the number of func-tional magnetic resonance imaging (fMRI) studies of Alzheimer’s disease (AD). The increasing popularity of fMRI is likely due to the tremendous need to find early markers for this devastating ill-ness, prior to the point of irreversible neuronal loss. The clinical course of AD is typically insidious, characterized by episodic mem-ory impairment beginning years prior to the time a clinical diagnosis is established. Evidence from postmortem and antemortem molecular imaging studies of subjects at risk for AD suggests that underlying pathology of AD may begin more than a decade prior to onset of dementia1. The link between the pathophysiological process of AD and the earliest clinical manifestations remains to be fully elucidated. Animal studies indicate that amyloid-related synaptic dysfunction precedes the onset of neuron loss and may account for early mem-ory deficits2,3; however, autopsy studies suggest that tangle pathology and neuronal loss may correlate better with clinical dementia severity at later stages of the disease4,5.
The lack of reliable diagnostic biomarkers, which can detect AD early in its course, continues to limit our ability to effectively develop and initiate disease-modifying therapies. In this respect, fMRI has the potential to elucidate the pathophysiological process that ultimately leads to AD and could prove to be an invaluable tool for early detec-tion of the disease and to assist in monitoring the effectiveness of new therapies.
This chapter will focus on recent findings from fMRI research on ageing and early AD, focusing on potential applications of fMRI for the diagnosis of AD and development of therapeutic agents.
POTENTIAL APPLICATIONS OF FUNCTIONAL IMAGING
Functional imaging techniques, in particular those that study brain activity during cognitive processes, have the potential to detect the earliest impact of pathophysiological changes on the functional integrity of neural networks. Although volumetric magnetic reso-nance imaging is presumably quite sensitive to loss of neurons and surrounding neuropil, it may not be able to detect changes until there is significant neuronal loss in specific medial temporal lobe (MTL) structures6, at a point too late to intervene with disease-modifying therapies. Functional imaging techniques that observe the brain at rest, such as fludeoxyglucose (18F) (18-fluorodeoxyglucose; FDG) positron emission tomography (PET), perfusion MRI7,8 and resting-state fMRI9,10, have shown evidence of regional abnormalities in early AD and may prove valuable in predicting subsequent decline in at-risk subjects. In order to detect brain dysfunction in individuals with very subtle cognitive impairment, and even asymptomatic individuals with early pathology of AD, we may need to employ more sensitive measures that probe the brain during exactly the types of cognitive processes that will become clinically impaired as the disease progresses. Task-related functional MRI may be able to detect alterations in the brain’s functional capacity prior to neuronal loss, or even evidence of dysfunction at rest.
The fMRI technique most widely used to investigate neural activity is based on imaging of the blood-oxygen-level-dependent (BOLD) contrast11. This depends on the magnetic properties of haemoglobin, which differ depending on whether or not it is bound to oxygen. The ratio between oxygenated haemoglobin, which is a diamagnetic sub-stance with zero magnetic moment, and deoxyhaemoglobin, which instead is a natural paramagnetic substance, in capillary beds and larger blood vessels subserving neural tissue creates an image con-trast detected by the MRI machine. Neuronal activity affects the ratio by increasing oxygen consumption and blood supply. Since the blood supply exceeds the oxygen demand, this results in an increase of the amount of deoxyhaemoglobin and therefore of the MR signal12. Thus, BOLD fMRI is an indirect measure of neuronal activity but can provide an important ‘window’ into the neural underpinnings of complex cognitive processes.
Figure 70.1 Functional MRI data during a face-name encoding paradigm. The top left figure demonstrates a statistical parametric map (SPM) of the hippocampus showing greater MR signal response to novel (N) face-name pairs compared to repeated (R) face-name pairs in a block-design fMRI paradigm. The top right shows a representative time course of the MR signal in the block-design paradigm, with marked increase in signal during novel blocks compared to blocks of visual fixation (+) or repeated blocks. The bottom left demonstrates significant hippocampal activation for an event-related face-name paradigm comparing successful vs. failed encoding. The bottom right graph shows the MR signal change in the right hippocampus for those face-name pairs correctly remembered with high confidence (yellow line), low confidence (pink line) or forgotten (blue line). A full colour version of this figure appears in the colour plate section
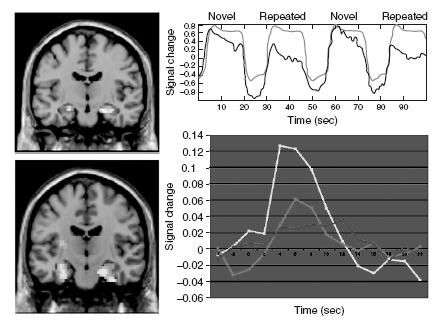
Typically BOLD fMRI is almost always reported as a ‘relative measure’, comparing the MR signal during one task (e.g. memory encoding) to a control task (e.g. viewing familiar stimulus) or a ‘baseline condition’ (e.g. visual fixation). Stimuli of each cognitive condition can be either grouped together in blocks lasting 20–40 s, as in a ‘block-design’ paradigm (see Figure 70.1), or can be interspersed with single stimuli of different conditions as in ‘event-related’ paradigms. The peak haemodynamic response is typically observed 4–6 s after the stimulus onset. Comparisons between con-ditions are performed using statistical packages that analyse the raw data of each voxel according to a general linear model (Statistical Parametric Mapping (SPM) and Brain Voyager are some commonly used analysis toolboxes). This type of analysis considers all the vari-ables that can affect signal within each voxel and ultimately translates in either increased BOLD signal, referred to as ‘fMRI activation’, or decreased BOLD signal, referred to as ‘fMRI deactivation’ in one condition compared to another. Deactivations or negative BOLD responses have been shown to reflect task-related decreases in neu-ronal activity below levels detected during spontaneous activity13, but it remains unclear whether this phenomenon is due to specific inhibitory influences.
At the present there is no way to determine an absolute measure of fMRI signal at ‘baseline’, and even resting fMRI scans investigate the correlations between regional MR signal over time to measure resting activity. Arterial spin labelling (ASL) perfusion fMRI techniques may provide a more robust baseline measurement of perfusion, but most task-related fMRI studies utilizing ASL techniques still rely on the ‘subtraction’ of MR signal in one condition versus another.
Intrinsic or resting connectivity studies examine the inter-regional correlations in the MR signal time course14.
In the future, information from multiple imaging modalities, partic-ularly perfusion MR, FDG-PET, and task-related BOLD fMRI, may be useful in determining whether the observed functional changes in patient populations relate primarily to alterations in ‘baseline’ func-tion vs. the neural requirements of the cognitive task, or perhaps to a combination of both factors.
A few studies have demonstrated fMRI alterations in sensorimotor areas in both cognitively normal older and AD patients15–17.These findings have invited caution in the interpretation of fMRI study results in ageing and AD, and raised concerns that changes in BOLD response could be due to a global decoupling of the haemodynamic response related to ageing and pathological processes of the vascular system.
Multiple studies have now demonstrated that the fMRI alterations in subjects with mild and moderate AD reflect regional changes spe-cific to the memory task and that the BOLD response in unaffected brain regions is quite similar in magnitude and extent to that seen in age-matched normal older control subjects18,19.
In recent years, fMRI studies have provided important insights into the neural underpinnings of the cognitive changes associated with ageing. Neuroimaging studies of non-demented older sub-jects compared to normal young adults have demonstrated group differences in the activation of brain regions responsible for the cognitive task in study. These findings have led to the formulation of the functional compensation theory and the dedifferentiation theory to explain the observed changes. The first theory postulates that decreased activation of brain regions in older compared to younger adults may represent less efficient processing. Instead, increased activity reflects compensatory mechanisms recruited to maintain efficient performance. Both PET and fMRI studies of memory performance have found loss of hemispheric asymmetry activation in older adults who have performances similar to those of young adults. Cabeza renamed this phenomenon ‘hemispheric asymmetry reduction in older adults’ (HAROLD). In one fMRI study of a word recognition task controlled for performance20,older participants showed increased activation of bilateral regions. In addition to increased bilateral activation to maintain performance, additional evidence points to age-related increased activation of the prefrontal cortex (PFC) associated with successful performance. This mechanism has been called ‘posterior–anterior shift in ageing’
Figure 70.2 The default network, a set of regions that demonstrate higher fMRI activity during rest compared to challenging cognitive tasks. In this group fMRI data map, regions in blue demonstrate greater MR signal during rest (visual fixation) compared to novelface-name encoding. A full colour version of this figure appears in the colour plate section
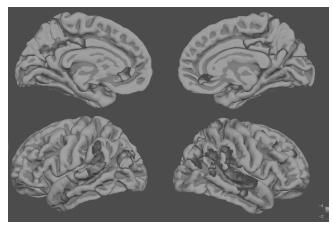
The adaptive nature of the changes described above is at the core of what Park and Reuter-Lorenz have termed the ‘scaffolding theory of ageing and cognition’ (STAC). They define scaffolding as ‘a process that results in changes of brain function through strengthening of existing connections, formation of new connections, and disuse of connections that have become weak or faulty’. In their view, both HAROLD and PASA can be conceptualized as part of the same compensatory process that reallocates tasks to preserved circuitries unaffected by ageing and possibly pathology to meet the task demands23.
tem’ that typically activates during memory retrieval tasks46,47.Our
The use of fMRI can be extended to investigate the brain networks involved in memory formation and how they are affected in AD. In particular, those studies using a ‘subsequent memory’ paradigm have demonstrated that greater fMRI activity in the MTL, as well as the left inferior prefrontal cortex, during encoding is associated with the likelihood of subsequent successful retrieval of the information24–28.This is further supported by the observation that compounds with known amnesic effects, such as benzodiazepines and anticholinergics, induce decreased activation in the hippocampus and prefrontal regions29,30.
Our own fMRI work has focused primarily on associative memory processes and, in particular, face-name associations. One primary role of the hippocampal formation in episodic encoding is to form new associations between previously unrelated items of information31,32. Learning the relationship between a name and a face is a particularly difficult cross-modal, non-contextual, paired associate memory task, and difficulty remembering proper names is the most common memory complaint of older individuals visiting memory clinics33,34. Tasks that tap into this cognitive process may be particularly useful in detecting the earliest memory impairment in AD1,35,36. Several fMRI studies, including our own in young subjects using a face-name associative encoding task27,29,37 show that associative memory paradigms produce robust activation of the anterior hippocampal formation38–40.
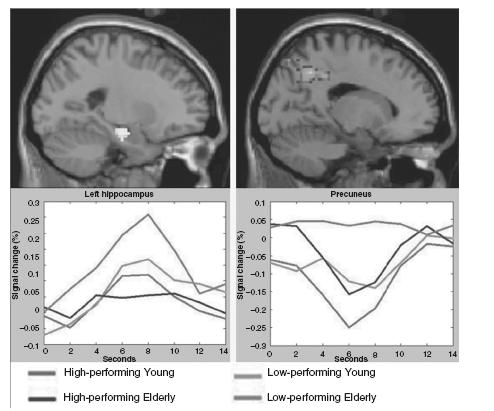
While activation in the anterior hippocampal formation is required for successful memory formation, a similar task-induced decrease in MR signal (i.e. deactivation), particularly in medial and lateral parietal regions, is also associated with successful encoding41,42. These lateral and medial parietal regions, including the precuneus and posterior cingulate, are central components of the ‘default-mode network’ (see Figure 70.2), characterized by Raichle and colleagues in a series of both PET and fMRI studies43,44. These regions typically exhibit increased activity at rest and decreased activity during externally focused task performance44. These parietal regions also appear to have significant connectivity with the hippocampus in resting-state network analyses14,45, and overlap with a ‘retrospenial memory system’ that typically activates during memory retrieval tasks46,47. Our own work in cognitively normal young and older adults suggests that the MTL system and the default-network system can be conceptualized as the ‘yin–yang’ of a distributed memory network. Successful encoding appears to require coordinated activity between these components, such that the lateral and medial parietal regions need to deactivate, while the MTL regions are activated42,48 (Figure 70.3).
fMRI STUDIES IN PATIENTS WITH CLINICALLY DIAGNOSED ALZHEIMER’S DISEASE
Since episodic memory impairment is an early finding of AD, the majority of fMRI studies to date have focused on episodic memory tasks18,19,49–57. These studies have employed a variety of unfamiliar visual stimuli, including faces17,49, face-name pairs19,55, scenes54, line-drawings50,57, geometric shapes51 and verbal stimuli56, and have consistently reported decreased hippocampal and parahippocampal activity in AD patients compared to healthy older controls during the encoding of novel stimuli. In our own studies using the blockdesign face-name paradigm, we found evidence that AD patients demonstrate significantly less hippocampal activation in the novelvs. repeated comparison than normal older controls19,53. Instead, during repeated stimuli exposure, AD patients continue to demon-strate increased MTL activation to stimuli that are highly familiarized to normal older controls58, and the degree of hyperactivation relates to poor post-scan memory performance. These findings indicate that intact MTL response suppression is an integral part of success-ful memory encoding and consolidation process and that failure of repetition suppression may be a sensitive marker of hippocampal dysfunction early in the course of AD59. Interestingly, in addition to MTL hyperactivation, several fMRI studies have also found evidence of increased activation in frontal and parietal regions in AD patients compared to controls, which, as described before, may represent a compensatory process in the setting of hippocampal failure19,55,56,60.
Figure 70.3 Group fMRI data maps (top) with MR signal time courses (bottom) for Young and Elderly controls show hippocampal activation (left) and precuneus deactivation (right) during the successful encoding of face-name pairs. Low-performing Elderly failed to deactivate the precuneus and demonstrated increased hippocampal and prefrontal activation for successful but not failed encoding trials, perhaps as a compensatory response to failure of default network activity. A full colour version of this figure appears in the colour plate section
Abnormal patterns of fMRI activity have also been found in the default-mode network regions of AD patients17,47,61,62. Buckner et al.47 were the first to note that these regions are strikingly similar to those regions that typically demonstrate evidence of fibrillar amyloid deposition binding with Pittsburgh compound B (PiB) in PET studies in AD63, as well as to the pattern of hypometabolism found on FDG-PET studies of AD patients64–66 and subjects at-risk for AD67–69
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






