Chapter 3 Functional neuroanatomy of the basal ganglia
Introduction
The basal ganglia comprise a collection of nuclear structures deep in the brain and have been defined anatomically and functionally. Anatomically, the basal ganglia are the deep nuclei in the telencephalon. Functionally, three closely associated structures, the subthalamic nucleus (in the diencephalon), the substantia nigra and pedunculopontine nucleus (both in the mesencephalon), are also included as part of the motor part of the basal ganglia. The definition of which structures are included has varied over the years and depends also in part on a preconceived notion of their function. Most of the time, and for the purposes of the study of movement disorders, the basal ganglia are viewed as having primarily a motor function. Indeed, the early movement disorders included in the concept, such as Parkinson disease (PD) (see Table 3.1 for all abbreviations in this chapter) and Huntington disease (HD), were primarily basal ganglia related, and interested neuroscientists would meet at “basal ganglia clubs.” It is now clear, however, that the basal ganglia also play a role in cognitive, behavioral, and emotional functions. For example, the limbic system interacts extensively with the basal ganglia, and some components of the basal ganglia, such as the amygdala (archistriatum), nucleus accumbens, and ventral pallidum, serve these functions (Haber and Knutson, 2010).
| AAADC | Aromatic L-amino acid decarboxylase |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| ADP | Adenosine diphosphate |
| AMPA | α-Amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| ATP | Adenosine triphosphate |
| BuChE | Butyrylcholinesterase (pseudocholinesterase) |
| cAMP | Cyclic adenosine monophosphate |
| ChAT | Choline acetyltransferase |
| CM | Centrum medianum nucleus of the thalamus |
| COMT | Catechol-O-methyltransferase |
| DA | Dopamine |
| DAG | Diacylglycerol |
| DAT | Dopamine transporter |
| DBH | Dopamine beta-hydroxylase |
| DBS | Deep brain stimulation |
| DOPAC | 3,4-Dihydroxyphenylacetic acid |
| EAAT | Excitatory amino acid transporter |
| GABA | Gamma-amino butyric acid |
| GABA-T | GABA-transaminase |
| GAD | Glutamic acid decarboxylase |
| GAT | GABA transporter |
| Glu | Glutamate |
| GP | Globus pallidus |
| GPe | Globus pallidus externa |
| GPi | Globus pallidus interna |
| HD | Huntington disease |
| 5-HT | 5-Hydroxytryptamine, serotonin |
| 5-HTP | 5-Hydroxytryptophan |
| HVA | Homovanillic acid |
| IP3 | Inositol triphosphate |
| LC | Locus coeruleus |
| L-dopa | Levodopa |
| LFP | Local field potential |
| M1 | Primary motor cortex |
| MAO | Monoamine oxidase |
| mAChR | Muscarinic acetylcholine receptor |
| MEA | Midbrain extrapyramidal area |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRN | Median raphe nucleus |
| 3-MT | 3-Methoxytyramine (3-O-methydopamine) |
| nAChR | Nicotinic acetylcholine receptor |
| NE | Norepinephrine |
| NMDA | N-methyl-D-aspartic acid |
| PD | Parkinson disease |
| Pf | Parafascicular nucleus of the thalamus |
| PMv | Premotor cortex, ventral division |
| PPN | Pedunculopontine nucleus |
| PPNc | Pedunculopontine nucleus, pars compacta |
| PPNd | Pedunculopontine nucleus, pars dissipatus |
| SERT | Serotonin transporter |
| SMA | Supplementary motor area |
| SN | Substantia nigra |
| SNc | Substantia nigra, pars compacta |
| SNr | Substantia nigra, pars reticulata |
| STN | Subthalamic nucleus |
| TANs | Tonically active neurons |
| TH | Tyrosine hydroxylase |
| VA | Ventral anterior nucleus of thalamus |
| VAChT | Vesicular ACh transporter |
| VL | Ventral lateral nucleus of thalamus |
| VMAT2 | Vesicular monoamine transporter 2 |
| VTA | Ventral tegmental area |
| ZI | Zona incerta |
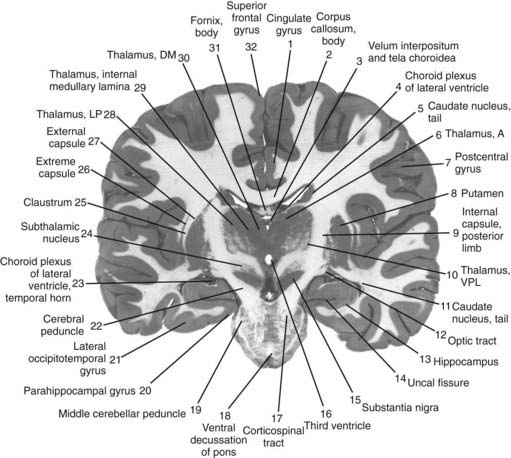
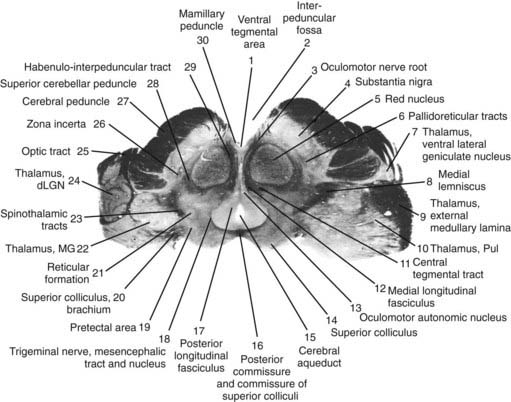
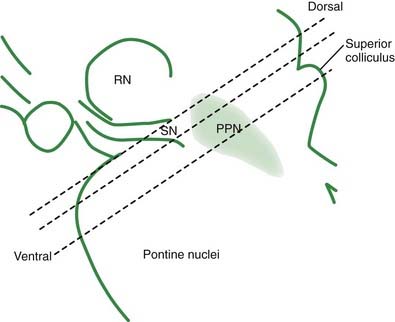
The core motor structures of the basal ganglia include the caudate and putamen, collectively called the neostriatum (commonly abbreviated as the striatum), the globus pallidus (GP) (paleostriatum), the subthalamic nucleus (STN), the substantia nigra (SN), and the pedunculopontine nucleus (PPN) (Figs 3.1, 3.2, and 3.3). The putamen and globus pallidus together are sometimes called the lenticular nucleus. The main informational processing loop of the basal ganglia comes from the cortex and goes back to the cortex via the thalamus. The substantia nigra pars compacta (SNc) is largely a modulator of this main loop, with dopamine as its neurotransmitter. Other modulators are the locus coeruleus (LC), with norepinephrine as neurotransmitter, and the median raphe nucleus (MRN), which uses serotonin as neurotransmitter. The notion that the basal ganglia provide an “extrapyramidal” control of movement separate from the cortical-pyramidal control is not correct since the main output of the basal ganglia projects to the cortex. Therefore, the term “extrapyramidal disorders” for disorders arising from dysfunction of the basal ganglia is a misnomer.
Neurotransmitters
Dopamine (DA)
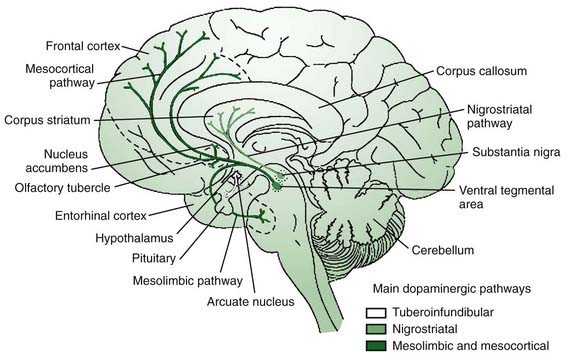
It is appropriate to start out the discussion of neurotransmitters with a consideration of dopamine, the most “prominent” neurotransmitter since it is depleted in PD and because we have the means to manipulate this transmitter in therapeutics. The main sources of dopamine are the lateral SNc (A9), the medial ventral tegmental area (VTA, A10), and the retrorubral area (A8) (Fig. 3.2). The SNc innervates the striatum via the nigrostriatal pathway, while the VTA and retrorubal areas give rise to the mesolimbic innervation of the ventral striatum (nucleus accumbens) and the mesocortical innervation of the dorsolateral and ventromedial prefrontal cortex regions (Fig. 3.4) (Van den Heuvel and Pasterkamp, 2008).
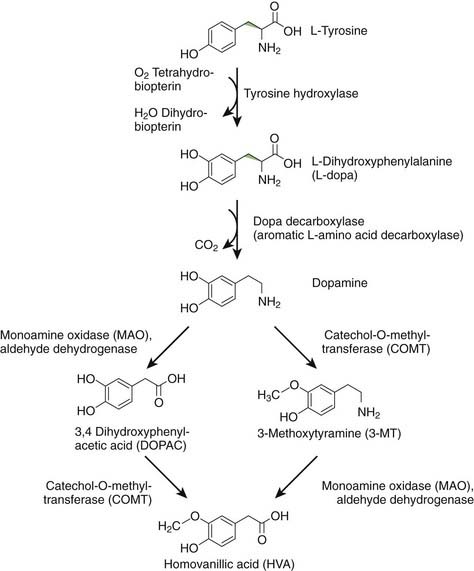
DA is formed from levodopa (L-dopa) by the enzyme aromatic L-amino acid decarboxylase (AAADC), which is commonly called dopa decarboxylase (Fig. 3.5) (Stahl, 2008). Once synthesized, DA is taken up into synaptic vesicles by the vesicular monoamine transporter 2 (VMAT2). In vivo, levodopa is synthesized from L-tyrosine by the enzyme tyrosine hydroxylase (TH). L-tyrosine is an essential amino acid in the brain, because it cannot be synthesized from L-phenylalanine, as it can in the rest of the body. DA can be metabolized by monoamine oxidase (MAO) to 3,4-dihydroxyphenylacetic acid (DOPAC), by catechol-O-methyltransferase (COMT) to 3-methoxytyramine (3-MT) (also called 3-O-methydopamine), and by both enzymes serially to homovanillic acid (HVA). MAO exists in two forms, MAO-A and MAO-B, both found in the mitochondria of neurons and glia (Bortolato et al., 2008). COMT is a membrane-bound enzyme (Bonifacio et al., 2007). Physiologically, DA action is terminated by reuptake back into the dopaminergic nerve terminal by action of the dopamine transporter (DAT). Once in the cytosol, it can be taken back up into synaptic vesicles by VMAT2. Dopamine neurons have MAO-A (Demarest et al., 1980), but virtually no COMT. DA not taken up into vesicles will therefore be metabolized to DOPAC. If DA remains non-metabolized in the cytosol, it might contribute to oxidative stress, as discussed in Chapter 5.
The exact biology of DA differs in different parts of the body and even different parts of the brain. For example, in the cerebral cortex there is not much DAT so that after DA release, COMT is much more important in terminating DA action (Matsumoto et al., 2003).
There are five subtypes of dopamine receptors, D1–D5, in two families, D1-like and D2-like (Missale et al., 1998; Beaulieu and Gainetdinov, 2011). The D1-like family, composed of D1 and D5, activates adenyl cyclase and causes conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Raising the concentration of cAMP is typically excitatory. The D2-like family, composed of D2, D3, and D4, inhibits adenyl cyclase and reduces the concentration of cAMP. Lowering cAMP is typically inhibitory. Some D2 receptors, called autoreceptors, are on the presynaptic side of dopamine synapses, regulating release by negative feedback.
Acetylcholine (ACh)
Cholinergic neurons have two different types of roles (Pisani et al., 2007). One is as an interneuron, and the “giant aspiny interneuron” of the striatum is cholinergic. A second role is as a projection neuron. There are two prominent cholinergic projection systems in the brain. The best known are the neurons of the basal forebrain, such as the nucleus basalis of Meynert, that innervate wide areas of cortex, are involved with functions such as memory, and are deficient in Alzheimer disease. The other is the set of projections from the meso-pontine tegmental complex, which includes the PPN. These are importantly involved in the basal ganglia motor system.
Acetylcholine is synthesized in neurons from choline and acetyl-CoA by the enzyme choline acetyltransferase (ChAT). After synthesis it is collected into vesicles by the enzyme vesicular ACh transporter (VAChT). Once released from the nerve terminals it is broken down by acetylcholinesterase (AChE), which is both pre- and postsynaptic, and butyrylcholinesterase (BuChE), also called pseudocholinesterase, that resides in glia (Cooper et al., 2003; Siegel et al., 2006). The resultant choline is taken back up into the presynaptic cell by a choline transporter (Stahl, 2008).
There are two broad classes of ACh receptors, nicotinic and muscarinic. Nicotinic receptors (nAChR) are ionotropic and are prominent outside the brain at the neuromuscular junction and autonomic ganglia, but are also in the brain (Albuquerque et al., 2009). Activation at an nAChR will open a nonselective cation channel allowing flow of sodium, potassium, and sometimes calcium. Muscarinic receptors (mAChR) are metabotropic and also found both inside and outside the brain. Activation at an mAChR couples to a variety of types of G proteins (Eglen, 2005, 2006). There are many types of nAChR and these are generally described by their subunit composition. Designations of M1–M5 are given to the mAChRs. Both nAChR and mAChR are found in the basal ganglia, and there are both excitatory and inhibitory effects.
Glutamate (Glu)
Glutamate is the primary excitatory neurotransmitter in the brain and as such it has a prominent role in the excitatory cortical-striatal input and in the excitatory projection from the STN to the globus pallidus interna (GPi). Glutamate is a central molecule in many cellular processes, and is also the precursor for the most important inhibitory neurotransmitter in the brain, GABA. Glutamate is made from glutamine in mitochondria by glutaminase. It is then taken up into synaptic vesicles by the vesicular glutamate transporter. Upon release, its action is terminated by its being taken up into glial cells via an excitatory amino acid transporter (EAAT) and then converted to glutamine by glutamine synthetase. Glutamine transporters then move the glutamine from the glial cell into the neuron (Siegel et al., 2006; Stahl, 2008).
Glutamate receptor biology is very complex and the details are well beyond this chapter. There are three groups of metabotropic glutamate receptors, groups I, II, and III, depending on mGluR composition. There are also three classes of ionotropic receptors, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), N-methyl-D-aspartic acid (NMDA), and the kainate (KA) receptors. Hence, glutamate not only transmits an excitatory signal by opening calcium channels, but also sets many metabolic processes in action, such as creating short- and long-term changes in synaptic excitability. Such changes are thought to be fundamental in brain plasticity (Lovinger, 2010).
Gamma-amino butyric acid (GABA)
There are three classes of GABA receptors: A, B, and C (Stahl, 2008). GABA-A and GABA-C are ionotropic, and have inhibitory action by opening chloride and potassium channels. There is much known about GABA-A, but only little about GABA-C. GABA-A channels have many subclasses depending on the subunit makeup. An important distinction between subclasses is whether they are sensitive to benzodiazepines or not, depending on whether the benzodiazepines bind to them or not. In the sensitive channels, benzodiazepines can increase the inhibitory action of a GABA-A synapse. GABA-B is a metabotropic receptor (Filip and Frankowska, 2008), and produces a longer duration inhibition than GABA-A by promoting potassium channels and inhibiting calcium channels.
Norepinephrine (NE)
There are a large number of NE receptors; the different classes are alpha 1A, 1B, 1D, alpha 2A, 2B, 2C, and beta 1, 2 and 3 (Stahl, 2008). All can be postsynaptic, and the alpha 2 receptors can also be presynaptic. Activation of the presynaptic receptors inhibits further NE release. The alpha 1 receptors are G protein coupled, and increase levels of phospholipase C, inositol trisphosphate (IP3), and calcium. The alpha 2 receptors are G protein coupled, with an action to inactivate adenylate cyclase and reduce concentrations of cAMP. The beta receptors couple to G proteins that activate adenylate cyclase and increase cAMP.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree