1 Fundamental concepts in functional neurology
Introduction
Much of the understanding that we have today of how human neurons function was based on the ‘integrate and fire’ concept formed by Eccles in the 1950s which was developed based on studies of spinal motor neurons (Brock et al. 1952). In this model, spinal motor neurons integrate synaptic activity, and when a threshold is reached, they fire an action potential. The firing of this action potential is followed by a period of hyperpolarisation or refraction to further stimulus in the neuron. This early integrate and fire model was then extrapolated to other areas of the nervous system including the cortex and central nervous system which strongly influenced the development of theories relating to neuron and nervous system function (Eccles 1951).
Early in the 1970s, studies that revealed the existence of neurons that operated under much more complex intrinsic firing properties started to emerge. The functional output of these neurons and neuron systems could not be explained by the existing model of integrate and fire for neuron function (Connor & Stevens 1971).
Central integrative state (CIS) of a neuron
The physical state of polarisation existing in the cell at any given moment is determined by the temporal and spatial summation of all the excitatory and inhibitory stimuli it has processed at that moment. The complexity of this process can be put into perspective when you consider that a pyramidal neuron in the adult visual cortex may have up to 12 000 synaptic connections, and certain neurons in the prefrontal cortex can have up to 80 000 different synapses firing at any given moment (Cragg 1975; Huttenlocher 1994).
‘And’ pattern neurons only fire an action potential if two or more specific conditions are met. ‘Or’ pattern neurons only fire an action potential when one or the other specific condition is present (Brooks 1984).
The thalamic relay cells exhibit complex firing patterns. They relay information to the cortex in the usual integrate and fire pattern unless they have recently undergone a period of inhibition. Following a period of inhibition stimulus, in certain circumstances, they can produce bursts of low-threshold spike action potentials referred to as post-inhibitory rebound bursts. This activity seems to be generated endogenously and may be responsible for production of a portion of the activation of the thalamocortical loop pathways thought to be detected in encephalographic recordings of cortical activity captured by electroencephalograms (EEG) (Destexhe & Sejnowski 2003).
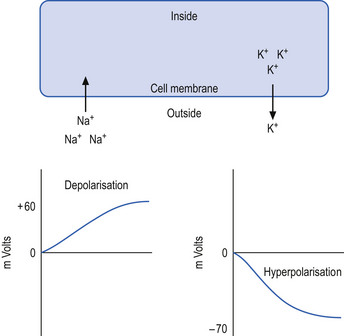
The neuron may be in a state of relative depolarisation, which implies the membrane potential of the cell has shifted towards the firing threshold of the neuron. This generally implies that the neuron has become more positive on the inside and the potential difference across the membrane has become smaller. Alternatively, the neuron may be in a state of relative hyperpolarisation, which implies the membrane potential of the cell has moved away from the firing threshold. This implies that the inside of the cell has become more negative in relation to the outside environment and the potential difference across the membrane has become greater (Ganong 1983) (Fig. 1.1).
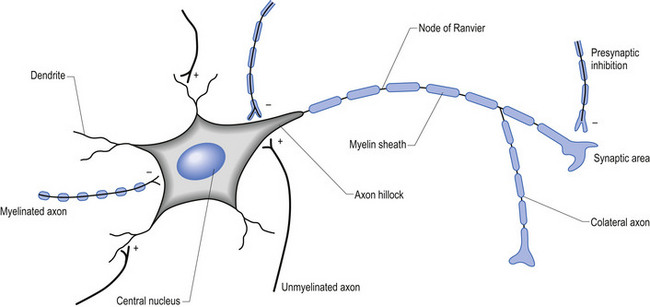
The firing threshold of the neuron is the membrane potential that triggers the activation of specialised voltage gated channels, usually concentrated in the area of the neuron known as the axon hillock or activation zone, that allow the rapid influx of Na into the axon hillock area, resulting in the generation of an action potential in the axon (Stevens 1979) (Fig. 1.2).
Transneural degeneration
1. Adequate gaseous exchange, namely oxygen and carbon dioxide exchange—this includes blood flow and anoxic and ischaemic conditions that may arise from inadequate blood supply;
2. Adequate nutritional supply including glucose, and a variety of necessary cofactors and essential compounds; and
3. Adequate and appropriate stimulation in the form of neurological communication, including both inhibition and activation of neurons via synaptic activation—synaptic activation of a neuron results in the stimulation and production of immediate early genes and second messengers within the neuron that stimulate DNA transcription of appropriate genes and the eventual production of necessary cellular components such as proteins and neurotransmitters.
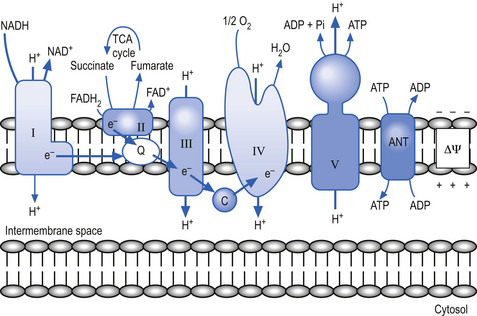
The mitochondria utilise a process called chemiosmotic coupling to harness energy from the food obtained from the environment for use in metabolic and cellular processes. The energy obtained from the tightly controlled slow chemical oxidation of food is used by membrane-bound proton pumps in the mitochondrial membrane that transfer H ions from one side to the other, creating an electrochemical proton gradient across the membrane. A variety of enzymes utilise this proton gradient to power their activities including the enzyme ATPase that utilises the potential electrochemical energy created by the proton gradient to drive the production of ATP via the phosphorylation of adenosine diphosphate (ADP) (Alberts et al. 1994). Other proteins produced in the mitochondria utilise the proton gradient to couple transport metabolites in, out of, and around the mitochondria (Fig. 1.3).
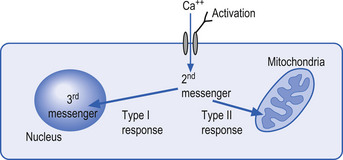
The mechanisms by which extracellular signals communicate their message across the neuron membrane to alter the protein production are discussed in Chapter 3. Here it will suffice to say that special transmission proteins called immediate early genes (IEG) are activated by a variety of second messenger systems in the neuron in response to membrane stimulus (Mitchell & Tjian 1989). Type 1 IEG responses are specific for the genes in the nucleus of the neuron and type 2 IEG responses are specific for mitochondrial DNA (Fig. 1.4).
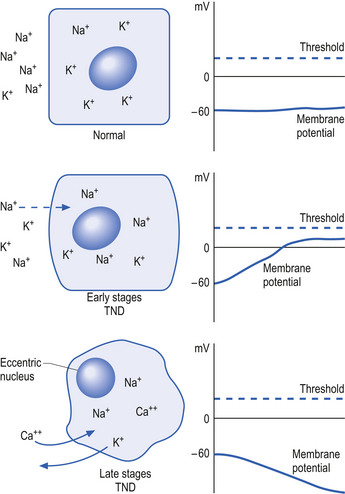
Initially, the neuron response to this down-regulation is to increase its sensitivity to stimulus so that less stimulus is required to stimulate protein production. This essentially means that the neuron alters its membrane potential so that it is closer to its threshold potential; in other words, it becomes more depolarised and becomes more irritable to any stimulus it may receive.
After a period of time if the neuron does not receive the deficient component in sufficient amounts, it can no longer sustain its state of depolarisation and starts to drastically downgrade the production of protein as a last ditch effort to conserve energy and maintain survival. At this stage, the neuron will still respond to stimulus but only for short periods as it consumes its available protein and ATP stores very quickly. In this state the neuron is vulnerable to overstimulation that may further exhaust and damage the neuron (Fig. 1.5).
The process of transneural degeneration may be one approach that determines the survival or death of neurons during embryological development where it has become quite clear that neurons that do not receive adequate stimulus do not usually survive (see Chapter 2).
Time to activation of a neuron or neuron system
The time to activation (TTA) of a neuron is a measure of the time from which the neuron receives a stimulus to the time that an activation response can be detected. Obviously, in clinical practice the response of individual neurons cannot be measured but the response of neuron systems such as the pupil response to light can be. As a rule, the TTA will be less (faster) in situations where the neuron system has maintained a high level of integration and activity and greater (slower) in situations where the neuron has not maintained a high level of integration and activity or is in the late stages of transneural degeneration. Again, an exception to this rule can occur in situations where the neuron system is in the early stages of transneural degeneration and is irritable to stimulus and responds quickly. This response will be of short duration and cannot be maintained for more than a short period of time.
Constant and non-constant neural pathways
Constant stimulus pathways are neural receptive systems that supply constant input into the neuraxis that are integrated throughout all multimodal systems to provide the stimulus necessary for the development and maintenance of the systems. Examples of constant stimulus pathways include receptors that detect the effects of gravity or constant motion, namely the joint and muscle position receptors of joint capsules and muscle spindles of the midline or axial structures including the ribs and spinal column. Certain aspects of the vestibulocerebellar system receive constant input and are constantly active. Several neural systems contain groups of neurons that exhibit innate pacemaker depolarisation mechanisms such as cardiac pacemaker cells, certain thalamic neurons, and selective neurons of the basal ganglia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree