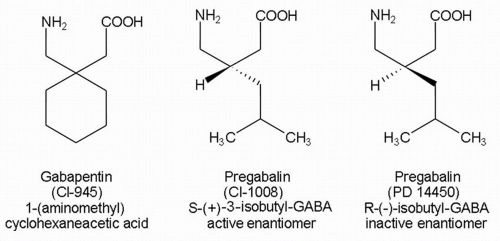
Gabapentin is an amorphous crystalline substance with a molecular weight of 171.24. It is freely soluble in water (
1,
4). The structure of gabapentin combines an inhibitory amino acid, GABA, and a cyclohexane ring that result in a zwitterion at physiologic pH (
1,
4). The agent is actively transported between body compartments by the L-system amino acid transporter, which recognizes such naturally occurring, bulky, neutral amino acids as L-leucine, L-isoleucine, L-valine, and L-phenylalanine. The same carrier is presumed to mediate transport across the gut wall, the blood-brain barrier, and cell membranes (
5,
6). Gabapentin concentrations can be measured in protein-free plasma samples by high-performance liquid chromatography (
7,
8) and gas chromatography (
9). Blood level assays are commercially available. Gabapentin degrades slowly to a lactam in solution as a function of pH, temperature, and buffer concentration (
10). The lactam is formed during synthesis of gabapentin, and has both proconvulsant (
11) and neuroprotective (
12) properties in laboratory models. Presumably, the lactam does not accumulate in sufficient quantity to be clinically significant. The current proprietary synthetic methods result in a low-lactam product (
13).