Fig. 3.1
Age-specific incidence of generalized-onset (solid circles) and partial-onset (plus signs) unprovoked epilepsies based on data collected in the Rochester Epidemiology Project from 1935 to 1984 (Hauser et al., 1993) Used with permission from Epilepsia
Unique Characteristics of Epilepsy in Elderly Patients
Types of Seizures
While the incidence of both focal and generalized unprovoked epilepsy increases in older adults, the most dramatic increase is in focal epilepsy (Hauser, Annegers, et al., 1993). In those over age 65, focal seizures with alteration of consciousness are the most frequent seizure type (48 %), followed by generalized (29 %) and focal seizures without alteration of consciousness (13 %) (Hauser, 1992). In elderly patients with chronic rather than new-onset epilepsy, seizures may become briefer and less elaborate over time, and generalized tonic-clonic seizures may become less frequent or even disappear (Tinuper, Provini, et al., 1996). Whereas focal seizures most often arise from the temporal lobe in the general population, these seizures in elderly patients often originate from extratemporal or frontal regions frequently affected by stroke (Ramsay, Rowan, et al., 2004).
Etiology of Seizures
Cerebrovascular disease is the most common non-idiopathic etiology of new-onset epilepsy in those aged 65 and older, accounting for 28 % of cases (Hauser, Annegers, et al., 1993). Approximately 20 % of cases were attributed to degenerative diseases, and less than 5 % were related to CNS tumors, trauma, or infection. Etiology remains unknown in 25–50 % (Hauser, Annegers, et al., 1993; Ramsay, Rowan, et al., 2004). See Fig. 3.2 for a comparison of etiologies of newly diagnosed epilepsy across the life span.
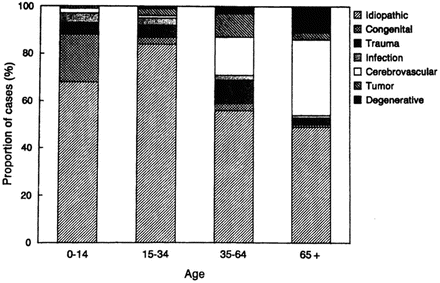

Fig. 3.2
Proportion of cases of newly diagnosed epilepsy assigned to specific etiologic categories within age groups, including idiopathic/cryptogenic category. Area: idiopathic (gray cross-hatched), congenital (dashed), trauma (dotted), trauma (widely dotted), infection (hatched), cerebrovascular (closely dotted), tumor (black), degenerative (light cross-hatch)
Diagnostic Complications
Diagnosing epilepsy in elderly patients can be complicated, and there is increased risk of both over- and underdiagnosis in this group. It is important for neuropsychologists conducting evaluations with older adults to keep this in mind, since presenting symptoms may reflect undiagnosed seizures. Older adults who presented with tonic-clonic seizures were correctly diagnosed 66.7 % of the time, although only 25.4 % of those with focal seizures without alteration of consciousness received an initial correct diagnosis (as described in Ramsay, Rowan, et al. (2004)). In patients with TIAs or strokes, the diagnosis of seizures was almost always delayed. It took an average of 1.7 years before correct diagnosis was made in this sample. This diagnostic difficulty is based, in part, on the limited knowledge of seizure semiology in the elderly, the reduced frequency of interictal discharges, the variety of EEG patterns seen, and the increased incidence of other conditions that may mimic seizures in this population (Van Cott, 2002). Further, it may be difficult to understand the presence or characteristics of spells in elderly individuals because patients are often more socially isolated (i.e., are more likely to live alone, be unemployed, have fewer social activities) and may have more memory problems than their younger cohort.
Because seizures tend to originate from extratemporal foci more often among elderly patients, they are less likely to exhibit the typical clinical manifestations characteristic of temporal lobe seizures (Ramsay & Pryor, 2000). Seizure symptoms are often nonspecific and may include altered mentation, staring, unresponsiveness, blackouts, and auras of dizziness (Ramsay & Pryor, 2000). Postictal confusion may persist for several days in older patients, compared to minutes in younger patients (Cloyd, Hauser, et al., 2006; Sheth, Drazkowski, et al., 2006).
These nonspecific symptoms make seizures more difficult to diagnose based on description alone. Several types of disorders or events may mimic seizures in elderly patients. Focal seizures with alteration of consciousness may be mistaken for TIAs or other cardiovascular disease, syncope, dementia, arrhythmias, or fluctuations in blood pressure or blood sugar levels (Sheorajpanday & De Deyn, 2007). The most common initial diagnoses in patients in the VACS 428 study whose diagnoses were later confirmed as epilepsy included altered mental status (41.8 %), confusion (37.5 %), blackout spells (29.3 %), and syncope (16.8 %) (Brodie & Kwan, 2005; Ramsay, Rowan, et al., 2004). See Table 3.1 for the primary differential diagnosis of seizures in elderly patients.
Table 3.1
Main differential diagnosis of seizures in elderly patients
Neurological |
– Transient ischemic attack |
– Transient global amnesia |
– Migraine |
– Restless leg syndrome |
– Dyskinesia |
Cardiovascular |
– Vasovagal syncope |
– Orthostatic hypotension |
– Cardiac arrhythmias |
– Structural heart disease |
– Carotid sinus syndrome |
Endocrine/metabolic |
– Hypoglycemia |
– Hypocalcemia |
– Hypomagnesemia |
Sleep disorders |
– Obstructive sleep apnea |
– Narcolepsy |
– Rapid eye movement sleep disorders |
– Hypnic jerks |
Psychological |
– Nonepileptic psychogenic seizures |
Status epilepticus (SE) often presents with no convulsive activity in elderly patients and may appear simply as confusion or minimal motor movements. In a prospective study, nonconvulsive SE was diagnosed in 16 % of elderly patients presenting with confusion of unknown origin (Baxendale, 1998). Thus, SE may go undiagnosed for several days in ambulatory elderly patients with ictal confusion (Sheth, Drazkowski, et al., 2006). This is concerning since even after it is treated, SE can result in persistent cognitive dysfunction. Further, SE is more common and has a higher morbidity rate in elderly patients (DeLorenzo, Hauser, et al., 1996).
Finally, further complicating an epilepsy diagnosis, EEG is less sensitive and specific for epilepsy in elderly patients (Brodie & Kwan, 2005). Absence of interictal epileptiform activity on routine EEG does not rule out the diagnosis, since its presence decreases with age and only occurs in 35 % of patients with preexisting epilepsy (mean age = 65) and 26 % of elderly patients with new-onset seizures (mean age = 70) (Drury & Beydoun, 1998). Conversely, benign EEG changes are associated with normal aging and have the potential to be misinterpreted as indicating a seizure tendency (Van Cott, 2002). Therefore, video-EEG monitoring may be valuable in establishing the diagnosis (Brodie & Kwan, 2005; McBride, Shih, et al., 2002; Van Cott, 2002).
Cognition in Elderly Patients with Epilepsy
Effect of Age on Cognition in Epilepsy
Epilepsy is associated with cognitive impairment, the cause of which is often multifactorial and may include underlying seizure etiology, ictal and interictal neuronal discharges, AED side effects, and psychosocial confounds (Kwan & Brodie, 2001). This is no different in elderly patients with epilepsy (Caramelli & Castro, 2005) and several factors may increase the risk of cognitive dysfunction in this group.
First, there is a subset of elderly patients with epilepsy who have had epilepsy for many years. There is evidence for greater cognitive morbidity when epilepsy has been long-standing (Helmstaedter, Kurthen, et al., 2003; Hermann, Seidenberg, et al., 2006).
Second, even “healthy” aging is associated with decreased processing speed and fluid intelligence, likely resulting from neurophysiological changes including loss of synapses, neurons, neurotransmitters, and neuronal networks (Fillit, Butler, et al., 2002). Furthermore, normal aging often results in mild levels of cerebral atrophy, ventricular enlargement, hippocampal atrophy, and deposition of beta-amyloid peptide and neurofibrillary tangles (Smith & Rush, 2006). The presence of epilepsy potentially exacerbates these changes.
Third, both aging and epilepsy have been associated with increased incidence of dementia and other disorders and lifestyle factors that can cause cognitive dysfunction (Hermann, Seidenberg, et al., 2008b). Compared to population-based controls, individuals with epilepsy had an increased relative risk of being diagnosed with Alzheimer’s disease at least 1 year after epilepsy diagnosis, with relative risk values ranging from 1.2 to 4.0 (Breteler, van Duijn, et al., 1991). Hermann (2008b) makes a compelling case that chronic epilepsy has been associated with several risk factors for poorer cognitive aging. As a group, those with chronic epilepsy have greater vascular risk factors, including more ischemic heart disease, hypertension, heart failure, diabetes, and cerebrovascular disease (Gaitatzis, Carroll, et al., 2004; Tellez-Zenteno, Matijevic, et al., 2005). This may be partially attributable to side effects (e.g., metabolic disorders, increased homocysteine) associated with select AEDs such as valproic acid and enzyme-inducing medications (Hamed & Nabeshima, 2005; Isojarvi, Rattya, et al., 1998; Luef, Waldmann, et al., 2004; Ono, Sakamoto, et al., 1997; Pylvanen, Knip, et al., 2003; Schwaninger, Ringleb, et al., 2000; Sheth, 2004). Elderly patients with epilepsy who had no preexisting cerebrovascular disease were at 2.89 times the risk of experiencing a first ever stroke compared to an elderly control group (Cleary, Shorvon, et al., 2004). Epilepsy has also been associated with increased inflammatory markers, both through the effects of seizures themselves (Vezzani & Granata, 2005) as well as AED effects (Verrotti, Basciani, et al., 2001). Finally, Hermann (2008) describes lifestyle factors which are both associated with poor cognitive aging and epilepsy, including decreased social networks and physical activity (Bjorholt, Nakken, et al., 1990; Nakken, 1999).
Although the above factors raise concern that elderly patients with epilepsy may be at greater risk of cognitive dysfunction than younger patients with epilepsy or older individuals without epilepsy, there has been little research published in this area. Older adults with chronic epilepsy performed more poorly across most cognitive measures compared to healthy older controls, both in a sample of medically intractable patients (Martin, Griffith, et al., 2005) and a sample in which 63 % were successfully controlled with medications (Piazzini, Canevini, et al., 2006). Griffith and colleagues (2006) found that memory deficits in older adults with chronic epilepsy were similar to deficits in patients with amnestic mild cognitive impairment, and the patients with epilepsy had greater difficulty on measures of executive functioning. Further suggestive of memory impairment in this group, elderly patients with chronic epilepsy did not demonstrate practice effects on a measure of verbal memory at 2–3-year follow-up compared to healthy elderly controls tested over the same interval (Griffith, Martin, et al., 2007), suggesting that elderly epilepsy may be associated with failure to learn compared to healthy elderly subjects.
Although these studies support cognitive morbidity associated with epilepsy in elderly patients, no study has directly investigated if this cognitive dysfunction is any greater than that experienced in younger adults with epilepsy, and evidence for accelerated cognitive decline associated with epilepsy in older adults is mixed. A recent cross-sectional study by Helmstaedter and Elger (2009) suggested against accelerated deterioration of episodic memory with older age in patients with TLE. Examination of verbal learning and memory in 1,156 patients with TLE and 1,000 controls over the life span revealed that the slow linear decline in verbal learning exhibited by normal controls after age 25 was mirrored in patients with TLE (although at a much lower level). Thus, although the TLE group had a lower level of performance, the rate of decline was the same between the groups. There was no relative decrease in memory in the TLE group compared to controls with advancing age. However, although patients ranged in age from 6 to 70 years, most were 50 years and younger, with only 17 patients over 60 years of age.
Conversely, there are other data supporting the possibility of age-accelerated cognitive decline in select patients with epilepsy. Another cross-sectional study by this same group suggested an age-accelerated decline in the retention of auditory information in preoperative patients who had temporal lobe epilepsy not confined to mesial temporal sclerosis (e.g., normal imaging or other lesions such as tumor) (Helmstaedter, Reuber, et al., 2002). Further, in a longitudinal study, Hermann et al. (2006) found that older age was one of the several predictors of decline in simple and complex psychomotor speed, but not other cognitive domains including memory, relative to expected values over a 4-year period in patients with chronic temporal lobe epilepsy. However, patients were relatively young in both of the above studies, with the average age in the low 30s.
In summary, there are several unique risk factors for cognitive morbidity in older adults with epilepsy. The few published studies examining the relationships among epilepsy, aging, and cognition suggest that older adults with epilepsy have more cognitive dysfunction than older adults without epilepsy and younger adults with epilepsy. It is unclear if this is due to additive or interactive effects of epilepsy and aging. However, most participants in these studies have been under 60 years old. More research is needed to determine the relationship between epilepsy and cognition as patients reach older age when the above comorbidities are more likely to start showing effects.
Epilepsy and Dementia
There is evidence for a bidirectional relationship between epilepsy and dementia. It is well established that those with dementia are at increased risk of developing epilepsy, and there is also some evidence that those with epilepsy may be at increased risk of developing dementia. Patients with dementia have a five- to tenfold increase in risk of seizures (Hesdorffer, Hauser, et al., 1996). Most of this research has been conducted in Alzheimer’s disease (AD), although there are a few studies reporting the presence of seizures in dementia with Lewy bodies (Weiner, Hynan, et al., 2003) and Creutzfeldt-Jakob disease (Marchiori, Yasuda, et al., 1996). It is estimated that between 10 and 22 % of patients with AD will experience at least one seizure (Mendez & Lim, 2003). The incidence of seizures increases with the severity of the dementia (Hesdorffer, Hauser, et al., 1996; McAreavey, Ballinger, et al., 1992; Mendez & Lim, 2003), with seizure onset often 7 years after dementia diagnoses (Mendez, Catanzaro, et al., 1994). However, seizures can begin at any time during the course of the illness (Hauser, Morris, et al., 1986), even as early as 3 months after diagnosis (Hesdorffer, Hauser, et al., 1996). The mechanism causing seizures in Alzheimer’s disease remains unknown, but suspected factors include the accumulation of amyloid-β plaques, neurofibrillary tangles, selective loss of inhibitory neurons, comorbid vascular lesions, and excessive neuronal cell loss in hippocampal and parietal cortices (Forstl, Burns, et al., 1992; Mendez, Catanzaro, et al., 1994; Mendez & Lim, 2003). The onset of seizures has been associated with a faster progression of cognitive and functional impairment in patients with AD (Volicer, Smith, et al., 1995).
There is also evidence that those with epilepsy may be at increased risk of developing a progressive dementia. Breteler et al. (1991) reviewed four studies that examined the relative risk of a subsequent diagnosis of Alzheimer’s disease in those with an epilepsy diagnosis. Diagnoses were based on interview with an informant in three studies and medical record review in the other. Compared to population-based controls, individuals with epilepsy had an increased relative risk of being diagnosed with AD at least 1 year after epilepsy diagnosis (relative risk values ranging from 1.2 to 4.0). The reason for the increased risk of subsequent AD diagnosis in those with epilepsy is unknown, although data from this study suggested that cumulative effects of long-standing seizures did not appear to be a factor in the development of dementia. The greatest risk for a diagnosis of AD was in patients who had epilepsy less than 10 years (relative risk = 2.4) versus 10 years or more (relative risk = 1.4). Alternatively, seizures may represent early pathological dementia-related changes in these patients, or the presence of both seizures and dementia may reflect shared risk factors. In a follow-up study, Breteler et al. (1995) found that patients diagnosed with epilepsy had a relative risk of 1.5 for being diagnosed with dementia over the next 8 years compared to other hospital patients. In an investigation of all patients diagnosed with probable AD by a neurologist over a 6-year period, 6.8 % had a history of epilepsy and/or were taking AEDs at the time of diagnosis (Lozsadi & Larner, 2006). In half of these cases, seizure onset occurred at about the same time as the onset of cognitive decline, with no identified acute cause of the seizures identified. Data from the Canadian Study on Health and Aging found that a diagnosis of epilepsy in those aged 65 years and older had a relative risk of 1.56 for being diagnosed with dementia over the next 5 years compared to community-dwelling controls without epilepsy, although this did not meet statistical significance (Carter, Weaver, et al., 2007).
Another way to determine whether epilepsy increases the risk of dementia is to compare brain tissue from epilepsy patients with tissue from patients and unaffected, age-matched controls. Mackenzie and Miller (1994) compared the number and location of senile plaques in temporal lobe tissue from epilepsy patients and normal controls. The age-related incidence of senile plaques was significantly higher in epilepsy patients. However, no patient showed any evidence of dementia on cognitive testing, and no other AD-related pathology was identified. Postoperative follow-up (mean 3.7 years, range 2–7) of the ten patients with senile plaques revealed no clinical suggestion of dementia, suggesting that the senile plaques were not associated with dementia or cognitive deterioration for at least several years (Mackenzie, McLachlan, et al., 1996). Overall, these findings suggest that TLE is associated with increased formation of senile plaques, but these plaques do not have an apparent effect on cognition, including the development of dementia.
Psychosocial Considerations in Elderly Patients with Epilepsy
Epilepsy can have profound effects on mood, anxiety, and quality of life (QOL). Elderly patients may be particularly vulnerable to these symptoms because they often live alone and may have additional physical and cognitive vulnerabilities that put them at increased risk for loss of independence.
The concern for worse QOL in the elderly compared to younger patients with epilepsy has not been born out in research findings. Comparisons between QOL measures in the elderly and younger adults suggest similar QOL in these groups (Baker, Jacoby, et al., 2001; Laccheo, Ablah, et al., 2008) or that older patients may even cope better with epilepsy than middle-aged peers (Pugh, Copeland, et al., 2005). A potential confounding factor is that QOL measures are generally developed and normed for those under 65 years of age. When Martin and colleagues (2005) gave a group of community-dwelling elderly adults a blank paper to list their concerns regarding living with epilepsy, results were similar in content to concerns voiced by younger epilepsy patients and included driving/transportation (64 %), medication side effects (64 %), personal safety (39 %), AED costs (29 %), employment (26 %), social embarrassment (21 %), and memory loss (21 %). Thus, the elderly with epilepsy may not have worse perceived QOL than younger patients but do report lower QOL than the general population (Laccheo, Ablah, et al., 2008) or older adults without epilepsy (McLaughlin et al. 2008).
Depression and anxiety rates are higher in patients with epilepsy in general, and this has been shown to extend to the elderly with epilepsy (Haut, Katz, et al., 2009). Further, many elderly people have been found to suffer from “subsyndromal” depression, in which they report significant depressive symptomatology but fail to meet formal diagnostic criteria for major depression (Strober & Arnett, 2009). Mental health disorders can be more difficult to diagnose in elderly patients with a neurological disorder compared to younger, otherwise healthy, individuals due to several factors including atypical presentation, difficulty distinguishing between symptoms of the mental health disease and epilepsy, and lack of assessment tools developed specifically for this population (Strober & Arnett, 2009). Nevertheless, it is often the responsibility of the neuropsychologist to determine whether or not a patient has a mental health disorder that requires treatment. It is important to understand unique characteristics of the presentation and treatment of mental health problems in the elderly with epilepsy. It has been suggested that depression in elderly individuals may present with more weight loss and fewer feelings of worthlessness and guilt than younger people (Frey, 2007), and depressive symptoms in patients with epilepsy may present as intermittent irritability, lack of energy, anxiety, and somatic symptoms such as pain (i.e., interictal dysphoric disorder). Many of the commonly used self-report inventories for depression do not have established cutoff scores for identifying depression in the elderly with epilepsy, although a recent review of depression assessment in the elderly with neurological disorders summarized the recommended cutoff scores for common measures when used with neurological populations (Strober & Arnett, 2009). The atypical presentation of depression in the elderly with epilepsy heightens the importance of a thorough clinical interview of the patient’s symptoms and lack of strict adherence to formal diagnostic criteria.
There is also little known about the most effective treatment for depression and other mental health disorders in the elderly with epilepsy. Cognitive behavioral and interpersonal are two therapy modalities that have received the most empirical support for treatment of depression (Barlow, 2001), although these studies have not specifically examined the elderly with epilepsy. Referral to a psychiatrist with specialized knowledge in treating emotional distress in those with epilepsy is often very helpful. The patient’s AED regimen should also be examined, since select AEDs have been associated with adverse mood side effects, most notably those such as phenobarbital, primidone, vigabatrin, topiramate, and levetiracetam (Frey, 2007). Finally, stressing medication compliance is especially important in this group, since depressed elderly medical patients were found to be less compliant with medications than nondepressed elderly medical patients (Carney, Freedland, et al., 1995).
Treatment of Epilepsy in Elderly Patients
AEDs
Epilepsy is more frequently controlled with AEDs in patients aged 65 years and over compared to younger patients (Mohanraj & Brodie, 2006). After starting AEDs for newly diagnosed epilepsy, approximately 80 % of patients aged 65 years and older remained seizure-free at 1-year follow-up (Brodie & Kwan, 2005).
Unfortunately, elderly patients are also generally more susceptible to the adverse effects of drugs than younger patients. Overall, AED side effects are more pronounced in elderly patients, there are more adverse drug interactions, and risk of toxicity is greater (Sheorajpanday & De Deyn, 2007). It is important for the neuropsychologist to be aware of situations in which AEDs may be contributing to cognitive deficits. Adverse side effects are more likely to occur with the specific AEDs described below, fast dose escalation rates, high doses, and polypharmacy (Sheorajpanday et al. 2007).
Despite these concerns, elderly individuals are underrepresented in AED clinical trials, and much of the information about AEDs used in elderly patients is derived from studies with younger adults (Beghi, Savica, et al., 2009). The few data available suggest that greater cognitive side effects of AEDs in elderly patients may be associated with polypharmacy (Griffith, Martin, et al., 2006; Piazzini, Canevini, et al., 2006) and old generation AEDs (Massimiliano, Rodolfo, et al., 2009). In addition, although cognitive side effects of topiramate have not been specifically examined in the elderly with epilepsy, there is reason to suspect that it carries cognitive risk based on the side effects seen in studies not restricted to the elderly (Sommer & Fenn, 2010).
Research has supported the effectiveness and tolerability of lamotrigine (LTG) and gabapentin (GBP) in elderly patients. Randomized controlled trials that have specifically recruited elderly patients found that LTG and/or GBP resulted in less early termination compared to carbamazepine (CBZ) with similar efficacy (Brodie, Overstall, et al., 1999; Rowan, Ramsay, et al., 2005; Saetre, Perucca, et al., 2007). An exception to this is one study that found no significant difference in effectiveness and tolerability of LTG and CBZ in elderly patients, although there were trends for greater tolerability of LTG and greater seizure-free rates of CBZ (Saetre, Perucca, et al., 2007). Several additional studies also found a good tolerability profile for, and effectiveness of, LTG in patients aged 60 or 65 and older (Arif, Buchsbaum, et al., 2010; Ferrendelli, French, et al., 2003; Giorgi, Gomez, et al., 2001; Mauri Llerda, Tejero, et al., 2005). Treatment guidelines from the International League Against Epilepsy state that, in focal epilepsies in elderly patients, LTG and GBP should be considered for initial monotherapy due to the available efficacy and effectiveness data (Glauser, Ben-Menachem, et al., 2006). Not all evidence is convergent on the superiority of LTG and GBP in elderly patients, however. A recent survey of patients aged 65–90 found no differences in adverse effects between LTG, CBZ, and sodium valproate (Brodie & Stephen, 2007), and Saetre et al. (2010) found that neither LTG nor CBZ caused significant changes in health-related quality of life. Other medications have not received as extensive study in the elderly with epilepsy, although oxcarbazepine appeared promising regarding tolerability in patients 65 years and older (Kutluay, McCague, et al., 2003).
Surgery
Epilepsy remains intractable to medications in approximately 20 % of elderly patients (Mohanraj & Brodie, 2006). Surgery is a successful treatment option for many patients with medically intractable epilepsy, and a recent study demonstrated similar rates of seizure outcome (i.e., Engel classes I and II) after temporal lobectomy with pathologically confirmed hippocampal sclerosis in those 50 and older (i.e., 95.2 %) compared with those under age 50 (i.e., 90.3 %) at follow-up approximately 10 years later. However, there has been some hesitancy to provide surgical treatment to elderly patients (Acosta, Vale, et al., 2008; Grivas, Schramm, et al., 2006; Sirven, Malamut, et al., 2000), in part due to concerns that surgery may exacerbate age-related cognitive decline (Grivas, Schramm, et al., 2006; Sirven, Malamut, et al., 2000).
The extent to which concern is warranted for poor cognitive outcome from surgery in elderly patients is unknown. Cross-sectional studies raise the possibility that temporal lobe surgery may accelerate memory decline with increasing age (Helmstaedter, Reuber, et al., 2002; Rausch, Kraemer, et al., 2003). Helmstaedter et al. (2002) found age-accelerated decline in postoperative verbal learning scores in patients after anterior temporal lobectomy compared to controls. This effect was not present in preoperative scores, in which age regression was similar to controls, suggesting that standard anterior temporal lobectomy has worse memory outcome for older individuals. However, there are sederal careats. First, age-related memory decline was specific to patients who underwent standard anterior temporal lobectomy for TLE that was not confined to mesial temporal sclerosis (e.g., normal imaging or other lesions such as tumor), as age-accelerated memory decline was not found in the selective amygdalohippocampectomy group with MTS. Second, older patients may have had a later age of epilepsy onset and longer duration of epilepsy, both of which were correlated with poorer memory, so results may, at least in part, reflect these factors. Finally, the average age of subjects in that study was 30 years, with no patient aged 60 or older. Similarly, Rausch et al. (2003) found that patients showed increasing deviation from age-corrected normative data on episodic auditory memory tests both 1 year and ~12 years after left temporal lobectomy. However, patients were rather young in this study as well, with an average age of 40 years at the long-term follow-up.
Two studies have been published to date that directly compare cognitive outcome in older versus younger patients after surgery for epilepsy. Both found that the older adults did not demonstrate greater memory declines than younger adults. Sirven et al. (2000) found no difference in memory change between 17 older (approximate age range 50–66) and 180 younger (approximate age range 18–49 years) patients before and after temporal lobectomy. Similarly, Grivas et al. (2006) did not find significant differences in memory changes between 34 older adults (approximate age range 50–71) and 359 younger adults (age <50 years) before and after temporal lobectomy. However, there was a trend for greater decline in postoperative auditory memory performance in the eight patients over 60 years of age than in those under 60 years of age.
Further, elderly patients appear to be at greater risk of cognitive decline after major surgery in general compared to younger adults, even when the surgery does not involve the heart or brain. A well-designed study by Monk and colleagues (2008) found that although about 31–40 % of non-demented adult patients experience cognitive dysfunction (i.e., deficits in episodic memory, executive functioning, and processing speed compared to control group) at hospital discharge with no difference between those who are young adult, middle aged, or elderly, the elderly patients were at significantly higher risk of cognitive deficits 3 months after surgery compared to young adults or middle-aged patients, as well as elderly controls. While prevalence of cognitive dysfunction in the younger groups returned to the levels of age-matched controls at 3 months after surgery (i.e., young adults 5.7 % and middle aged 5.6 %), the prevalence of cognitive dysfunction in the elderly group was significantly higher (12.7 %). Additional predictors of cognitive dysfunction at 3-month follow-up included cognitive dysfunction at hospital discharge, increased age, history of cerebral vascular accident with no residual impairment, and years of education. These results are similar to those of another similar large-scale study (Moller, Cluitmans, et al., 1998). Thus, older age appears to be a risk factor for cognitive dysfunction after major surgery in general.
In summary, the elderly may be at increased risk of postoperative cognitive decline after any major surgery. Regarding cognitive risk associated specifically with surgery for epilepsy, preliminary cross-sectional data suggest that anterior temporal lobectomy may accelerate age-related memory decline in patients with pathology other than MTS, although this increased cognitive risk for elderly patients has not been born out in direct comparisons of memory decline in older versus younger adults. However, the few available studies in this area have been limited by a small sample size of older patients, with few if any of the samples composed of those over age 60 years.
Methods
Role of the Neuropsychologist Assessing Elderly Patients with Epilepsy
There are several different reasons why a neuropsychologist may be asked to conduct an evaluation of an elderly patient with epilepsy. Referral questions can include those typical of a younger adult with epilepsy, such as characterizing the impact of seizures, AEDs, or surgery on cognition; obtaining a baseline of cognitive functioning by which to measure any changes in the future (e.g., after surgery, AED changes, or ongoing seizures); and providing insight into the level of cognitive functioning in order to help estimate functional abilities. Additionally, in the elderly with epilepsy, it may be important to determine whether or not the patient appears to be experiencing a comorbid progressive neurodegenerative process. Any neuropsychological evaluation for an elderly patient with epilepsy should take into account the unique etiology, cognitive considerations, psychosocial functioning, and treatment implications of this group that are outlined above.
Neuropsychologists are often asked to evaluate the cognitive functioning and comment on the potential etiology of deficits in elderly patients with episodes of confusion, memory complaints, and changes in behavior in the absence of epilepsy. It is important to be mindful of the possibility that seizures may be occurring in these patients presenting with rather nonspecific symptoms. Since there is a great deal of overlap between symptoms of dementia and epilepsy, careful history taking and evaluation of any focal area(s) of neurocognitive deficit are key features of the examination in order to determine whether seizures are a potential etiology that deserve further neurological investigation.
Appointment Scheduling
Elderly patients with epilepsy may require slight modifications of the assessment procedure. Obtaining a collateral report is particularly important when conducting a neuropsychological evaluation of elderly patients with epilepsy due to possible difficulty describing seizure-related details and unawareness of cognitive symptoms, particularly in the case of suspected progressive dementias. Elderly patients may become fatigued more quickly during testing, and a shorter battery is often required. Alternatively, in some settings, testing could be broken up into multiple sessions. It may be helpful to structure testing so that measures within a specific cognitive domain are spaced throughout the evaluation to avoid an entire domain being affected by fatigue towards the end of a testing session, as well as maximizing the chance of getting a variety of cognitive domains assessed even if the patient has to discontinue before the end of the evaluation. Scheduling testing for the morning is often preferred, since evidence suggests that older adults perform better on cognitive tasks in the morning than in the afternoon (Hasher, Chung, et al., 2002).
Interview
In addition to obtaining standard background information in a neuropsychological interview, when assessing the elderly with epilepsy, it is even more important to gain a complete and detailed understanding of the history of onset and progression of cognitive, behavioral, and mood symptoms and how these overlay any changes in seizures, AEDs, other medications and medical problems, and other relevant life events (e.g., retirement, death of a spouse). The information obtained in the interview is often the most valuable data for establishing a list of possible etiologies for subjective or objective cognitive impairment. Common etiologies of cognitive dysfunction to consider include:
Acute and chronic effects of seizures
AED or other medication side effects
Progressive dementia
Cerebrovascular disease
Head trauma
Sleep problems
Emotional distress
Other medical comorbidities, including any known cause of epilepsy
Cerebrovascular etiology or comorbid disease should be considered in all elderly patients with epilepsy, since cerebrovascular disease accounts for about one third of newly diagnosed cases of epilepsy in elderly patients. Furthermore, elderly patients with epilepsy are at a higher risk of experiencing a first ever stroke than the elderly without epilepsy (Cleary, Shorvon, et al., 2004). Similarly, evaluations should consider the presence of possible progressive dementias, since about 12 % of newly diagnosed cases of epilepsy in elderly patients are attributed to degenerative disorders, and some evidence suggests that epilepsy increases the risk of developing AD (Breteler, van Duijn, et al., 1991). Other conditions affecting cognition in older adults not listed above also need to be considered as comorbid conditions, although they are not necessarily associated with epilepsy specifically. These include, but of course are not limited to, Parkinson disease, normal-pressure hydrocephalus, hypothyroidism, vitamin B12 deficiency, thiamine deficiency, and sleep breathing disorders. Finally, it is also particularly important to assess activities of daily living, since this is helpful in differential diagnosis and forming recommendations.
Test Selection
General Considerations
Neuropsychological evaluation of any elderly patient requires special considerations. There are limited normative data on many common neuropsychological measures for people of advanced age, although this has improved over the past decade. Test selection should take into careful account the robustness of the norms for older adults. The MOANS and MOAANS studies in particular provide good normative data for individuals between the ages of 56 and 95 (e.g., Ivnik, Malec, et al., 1996; Lucas, Ivnik, et al., 2005). Increased frequency of motor and sensory deficits in this population requires understanding any limitations of the patient and adapting tests appropriately. For example, grooved pegboard performance may be affected by peripheral injury, and results would not reflect brain dysfunction per se. Unique mood, quality of life, and functional issues in elderly patients suggest the use of specific questionnaires developed for this population. Consideration should be given to measures targeting cognitive symptoms specific to common medical comorbidities in this population, such as dementia and cardiovascular disease, even if not directly related to the referral question to help track possible changes in the future (e.g., inclusion of a dementia screening measure). Finally, as in any neuropsychological evaluation, test selection should take into account the specific referral question. In the epilepsy population, measures to help localize and lateralize dysfunction should be included. Considerations for interpreting data in the context of the common referral questions listed above are addressed in the below section “Reporting the Findings.”
Domains Assessed
Based on the issues listed above, recommended tests are listed in Table 3.2. This is not meant to be an all-inclusive list of useful tests, nor is this meant to suggest that all tests need to be administered. Rather, this list is provided as a core of select measures useful in the elderly epilepsy population and could be modified based on the specific referral question, the clinician’s preliminary impression, and any restrictions posed by a patient such as fatigue or sensory/motor problems.
Table 3.2




Recommended tests for neuropsychological assessment of elderly patients with epilepsy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





