HIV associated asymptomatic neurocognitive impairment (ANI)
1. Acquired impairment in cognitive functioning, involving at least two ability domains, documented by performance of at least 1 standard deviation below the mean for age-education-appropriate norms on standardised neuropsychological tests. The neuropsychological tests must survey at least the following abilities: verbal/language, attention/working memory, abstraction/executive, memory (learning, recall), speed of information processing, sensory-perceptual, motor skills
2. The cognitive impairment does not interfere with everyday functioning
3. The cognitive impairment does not meet criteria for delirium or dementia
4. There is no evidence of another pre-existing cause for the ANI
HIV associated mild neurocognitive disorder (MND)
1. Acquired impairment in cognitive functioning, involving at least two ability domains, documented by performance of at least 1.0 standard deviation below the mean for age-education-appropriate norms on standardised neuropsychological tests. The neuropsychological tests must survey at least the following abilities: verbal/language, attention/working memory, abstraction/executive, memory (learning, recall), speed of information processing, sensory-perceptual, motor skills
2. The cognitive impairment produces at least mild interference in daily functioning (at least one of the following):
(a) Self-report of reduced mental acuity, inefficiency in work, homemaking or social functioning
(b) Observation by knowledgeable others that the individual has undergone at least mild decline in mental acuity with resultant inefficiency in work, homemaking or social functioning
3. The cognitive impairment does not meet criteria for delirium or dementia
4. There is no evidence of another pre-existing cause for the MND
1. Marked acquired impairment in cognitive functioning, involving at least two ability domains, typically the impairment is in multiple domains, especially in learning of new information, slowed information processing, and defective attention/concentration. The cognitive impairment must be ascertained by neuropsychological testing with at least two domains 2 standard deviations or greater than demographically corrected means
2. The cognitive impairment produces marked interference with day-to-day functioning (work, home life, social activities)
3. The pattern of cognitive impairment does not meet criteria for delirium
4. There is no existing evidence of another, pre-existing cause for the dementia
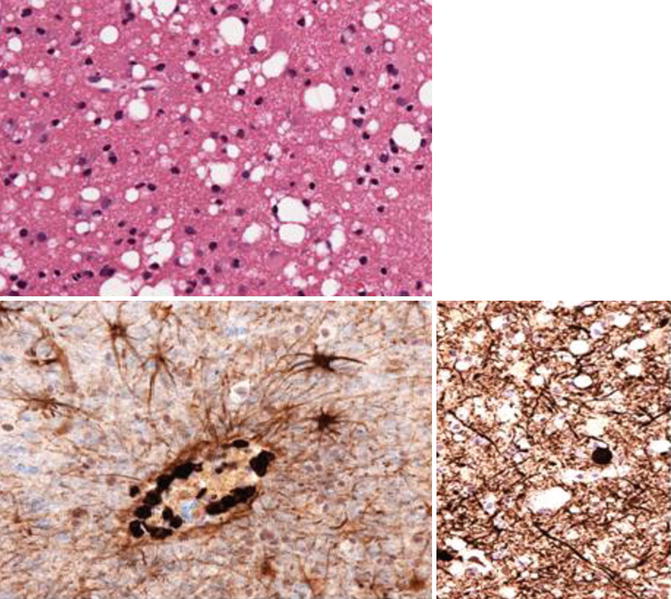
Fig. 14.1
Top: vacuolar leukoencephalopathy; bottom right: shows perivascular CD8 T lymphocytes, Bottom left: shows axonal injury; Histology slides courtesy of Prof Michael Farrell (Department of Neuropathology, Beaumont Hospital, Dublin, Ireland)
14.3 Prevalence of Cognitive Impairment in HIV
Prior to the introduction of HAART up to 20 % of patients with AIDS developed HIV dementia and it was associated with a high mortality rate with a mean survival of 6 months to 1 year after the development of dementia. Prior to HAART mild neurocognitive impairment was described in 30 % of patients with asymptomatic HIV disease and in up to 50 % of patients with AIDS defining illnesses. The incidence of HIV associated dementia has declined in the post-HAART era to approximately 2 %, however cognitive impairment continues to be an ongoing clinical issue despite good virological control of HIV. Prevalence rates of 20–50 % of HAND have been demonstrated in large prospective studies.
14.4 Neuropathology of HIV
HIV is neuroinvasive, neurotropic and neurovirulent. HIV enters the brain through infected monocytes and lymphocytes. They are used as a “Trojan horse” mechanism to cross the blood brain barrier. HIV has been found in the cerebrospinal fluid (CSF) of patients during seroconversion, suggesting that HIV enters the CNS early in the course of infection. Neurons are not infected directly by HIV. Microglial cells and macrophages are the primary targets for productive HIV infection within the CNS as they both possess receptors for CD4 and CCR5. Microglia cells act as the brain’s native immune response cells. Astrocytes are also infected by HIV despite their lack of receptors for viral attachment but in a more restricted fashion.
HIV can cause an inflammatory reaction upon entry to the CNS which may be manifested by a T cell reaction with vasculitis and leptomeningitis. There is upregulation of MHC class II antigens, increased number of microglial cells found and increased production of cytokines. Perivascular microglia are likely involved early in infection and the parenchymal microglia become involved in the later stages of infection. The chemokine receptor CCR5 is the dominant receptor used within the brain. There is proliferation of astrocytes and intense perivascular lymphocytic and macrophagic infiltration has been seen at autopsy which has been postulated to be related to immune reconstitution inflammatory syndrome (IRIS).
The pathognomonic feature of HIV encephalitis (HIVE) on histological examination is the presence of multinucleated giant cells. These cells are the fusion of infected and uninfected microglia and macrophages and they stain positively for HIV antigens. Together they provide evidence of productive infection by HIV within the CNS. This pathological feature was present in 20–50 % of patients who had autopsies performed in the first 15 years of the HIV epidemic. HIVE has a predilection for the basal ganglia and white matter and affects the neocortical grey matter, cerebellum and brainstem to a lesser extent. Other pathological features of HIV include myelin pallor, axonal loss, microglial nodules and gliosis. There is also disruption of the blood brain barrier and apoptosis of astrocytes. This leads to dendritic and neuronal loss (Fig. 14.2; Table 14.2).
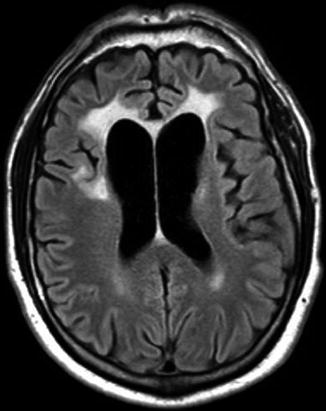
Table 14.2
Summary of neuropathogenesis
Neuropathology | |
Pathological findings | Lymphocytic meningitis |
Multinucleated giant cells with encephalitis | |
Perivascular lymphocytic and macrophagic infiltration | |
Myelin pallor | |
Axonal loss | |
Microglial nodules | |
Gliosis | |
Inflammatory and immune activation | Effects and roles |
Activated macrophages and monocytes | Release viral gene products and proinflammatory cytokines |
CD163 is a marker of activated macrophages | |
Expansion of CD14+/CD16+ monocytes | |
Migration of CD16+ cells across BBB increased by fractalkine | |
TNF + VCAM-1 increase membrane bound fractalkine | |
IFN-γ and ICAM-1 decrease membrane bound fractalkine | |
CD8+ T Cells | CD8 encephalitis |
Loss of specific cytotoxic CD8 immune response in aging | |
Microbial translocation | Increased LPS induces monocyte activation and trafficking into CNS |
LPS induces expression of E-selectin and VCAM-1 on macrophages | |
Neurotoxic and neurodegenerative mechanisms | Effects and roles |
Viral proteins | |
Gp120 | Toxicity of blood brain barrier |
Activates protein kinase C | |
Cytotoxicity requires presence of IFN-γ and p38 MAPK | |
Degradation of ZO-1 and ZO-2 | |
Dysfunction of nigrostriatal dopaminergic system | |
Alters glutamate signalling + Ca++ homeostasis | |
Tat | Affect tight junction proteins |
Decreases expression of occludin, ZO-1, ZO-2 | |
Increases trafficking of monocytes into CNS | |
Neuronal cell death and excitotoxicity – partially dependent on NMDA receptors | |
Neuronal apoptosis increased by tat and TNF-α | |
Mitochondrial dysfunction | |
Vpr | Disrupts Ca++ homeostasis |
Impairs glutamate signalling | |
Nef | Apoptosis of brain microvascular endothelial cells |
Causes neuronal damage mediated by MCP-1 | |
Platelet activating factor | Increases glutamate release |
Increases vulnerability of neurons to dendritic alterations | |
Neopterin | Elevated in CSF and remains mildly elevated even with viral suppression |
Induces release of QUIN | |
Correlates with release of reactive oxygen species by macrophages | |
QUIN | Excitotoxic agonist at NMDA receptors |
Forms free radicals | |
Correlates with cerebral atrophy | |
Monocyte chemoattractant protein 1 (MCP-1) | Upregulated in HIVE |
Associated with poorer outcomes | |
Mediates neuronal death caused by nef | |
Astrocytes | Astrocytosis – feature of HIVE |
Astrocyte apoptosis propagates macrophage activation and causes loss of neurotrophic support for neurons | |
Synaptodendritic injury | Retraction of dendritic spines, dendritic beading and aberrant sprouting |
Disrupts normal communication and impairs axonal transport | |
NFL is a marker of axonal injury | |
HAART | Mitochondrial dysfunction |
Beading and pruning of dendrites | |
Destabilisation of neuronal Ca++ homeostasis and a reduced response to glutamate | |
Increase NO production | |
Aging | Decreased levels of amyloid-beta |
Normal or low levels of tau | |
Neuritic α-synuclein expression in substantia nigra |

Fig. 14.2
Bifrontal white matter hyperintensities (seen on FLAIR) in a patient with HIV dementia and vacuolar leukoencephalopathy on the same patient’s brain biopsy. MRI courtesy of Dr Niall Sheehy (Department of Radiology, St James’s Hospital, Dublin, Ireland)
14.5 Risk Factors for HAND
Risk factors include a low nadir CD4+ T-cell count, older age, substance abuse, lower educational level, co-infection with hepatitis C, CCR2 polymorphisms, the 2578G variant of CCL2 (MCP-1), TNF-α 308A allele and systemic features such as low haemoglobin, constitutional symptoms and a low body mass index. Apolipoprotein E status, particularly in older patients, has been associated with HAND in some studies.
14.6 Functional Consequences of HAND
HIV-associated dementia remains an independent predictor of time to death. Functional impairment in the areas of work assessment, finances, medication management, cooking and shopping has been demonstrated. Unemployment is higher.
14.7 Clinical Features in the 1980s of HAND
Patients exhibited disturbance in cognitive, behavioural and motor function. Patients complained of forgetfulness, loss of concentration, difficulty recalling recent events and losing their train of thought. Motor symptoms included lower limb weakness, loss of balance and abnormal co-ordination. Apathy and social withdrawal were the most common behavioural symptoms. It was consistent with a subcortical dementia given the prominent psychomotor slowing and the absence of cortical signs such as aphasia and apraxia. The neuropathological findings supported this hypothesis given the predominance of white matter and subcortical nuclei pathology. One study demonstrated increased rates of neuropsychological impairment at each successive stage of HIV infection. The domains most often affected were attention, speed of information processing and learning efficiency and they suggested that this pattern was consistent with earliest involvement of subcortical or fronto-striatal brain systems.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








