Hormones, Catamenial Epilepsy, and Reproductive and Bone Health in Epilepsy
Martha J. Morrell
Steroid hormones alter the excitability of neurons of the cerebral cortex and thereby alter the seizure threshold. Seizures, in turn, change the endocrine environment, probably through actions on the hypothalamic-pituitary axis. Some antiepileptic drugs (AEDs) further complicate hormone-seizure interactions, altering the metabolism and binding of steroid hormones. This chapter discusses the clinical implications of these hormone-seizure interactions, focusing on the impact of ovarian steroid hormones on seizures and of epilepsy on reproductive and bone health.
EFFECTS OF STEROID HORMONES ON NEURONAL EXCITABILITY
Steroid and thyroid hormones influence brain excitability (1). This dynamic relationship is most clearly established for the ovarian sex steroid hormones estrogen and progesterone (2,3). Fluctuations in these hormones over a reproductive cycle change seizure susceptibility in experimental models of epilepsy (4,5).
Estrogen activates seizures in experimental models of epilepsy and in human cerebral cortex. Estrogen lowers the electroshock seizure threshold (6, 7, 8), creates new cortical seizure foci when applied topically (9), activates pre-existing cortical epileptogenic foci (10), and increases the severity of chemically induced seizures (11,12). Intravenously administered estrogen activates electroencephalographic (EEG) epileptiform activity in some women with partial epilepsy (13).
In contrast, progesterone exerts a seizure-protective effect in experimental models. High doses induce sedation and anesthesia in rats and in humans (14), primarily as a result of actions of the metabolite pregnenolone. Progesterone reduces spontaneous interictal spikes produced by cortical application of penicillin (15) and suppresses kindling (16) and focal seizures (17) in animals. It also heightens the seizure threshold to chemical convulsants (18,19), elevates the electroshock seizure threshold (8,20), and attenuates ethanol-withdrawal convulsions (18).
Steroid hormones modulate cortical excitability through several mechanisms of action. They bind to an intracellular receptor (intracytoplasmic for glucocorticoids, intranuclear for estrogen and progesterone). Binding transforms the receptor to an active form that binds to deoxyribonucleic acid (DNA), leading to gene activation and protein synthesis; this process requires 30 minutes to several hours to complete. Many neuroactive effects of steroids are evident in seconds to minutes, suggesting that some actions are also mediated at the neuronal membrane.
Gonadal and adrenocortical steroid hormones exert immediate, short effects on neuronal excitability by altering γ-aminobutyric acid (GABA)-mediated neuronal inhibition and glutamate-mediated excitation at the cell membrane (21,22). A sex steroid hormone-recognition site is present on recombinantly expressed GABAA-receptor
complex derived from human complementary DNA (cDNA) (23). Neurosteroids, such as those in the ovary, may act at two sites on the GABAA-receptor complex: directly on the chloride channel and at a distinct site that mediates the action of GABA and benzodiazepines (24,25).
complex derived from human complementary DNA (cDNA) (23). Neurosteroids, such as those in the ovary, may act at two sites on the GABAA-receptor complex: directly on the chloride channel and at a distinct site that mediates the action of GABA and benzodiazepines (24,25).
Ovarian steroids have opposing effects on neuronal excitability (26). Estrogen reduces the effectiveness of GABA-mediated neuronal inhibition by decreasing chloride conductance through the GABAA-receptor complex. Longer-latency effects of estrogen on neuronal excitability are exerted through inhibition of GABA synthesis in the arcuate nucleus, the ventromedial nucleus of the hypothalamus, and the centromedial group of the amygdala (27), probably through regulation of messenger ribonucleic acid (mRNA) encoding for glutamic acid decarboxylase (GAD), the rate-limiting enzyme for GABA synthesis (28,29). Estrogen also affects mRNA encoding for GABAA-receptor subunits (30). Estradiol rapidly increases responses of neurons to the excitatory neurotransmitter glutamate through agonist binding sites on the N-methyl-D-aspartate (NMDA)-receptor complex (31, 32, 33, 34) and through a G-protein-dependent mechanism on non-NMDA glutamate receptors that activate protein kinase (35).
Woolley and colleagues demonstrated the proconvulsant effect of estrogen (2,36). Three days’ exposure to elevated estradiol levels increased the density of dendritic spines and excitatory synapses on hippocampal neurons; the same also occurs with natural hormone fluctuations over the estrous cycle (37).
Progesterone and its metabolites function as allostericreceptor antagonists or inverse agonists at the GABAA-receptor complex (38), potentiating GABA-induced chloride conductance by increasing the frequency (benzodiazepinelike effects) and duration (barbiturate-like effect) of channel opening (39,40). Progesterone also modulates GAD (19), alters expression of mRNA encoding for GABAA-receptor subunits (29,30), and reduces glutamate activity (32,33).
The sensitivity of neurons to the modulating effects of individual steroid hormones changes after puberty and in response to fluctuations in basal levels of steroid hormones over a reproductive cycle (19,41). The pubertal surge in estrogen appears to have a neuronal priming effect. In contrast to its effects in postpubertal rats, estrogen does not alter the rate of amygdala kindling in prepubertal male and female rats. Rats castrated prepubertally have higher seizure thresholds to minimal and maximal electroshock than do animals castrated after puberty (8,42). Several rodent models of epilepsy suggest that the sensitivity of the GABAA-receptor complex to neurosteroids varies so as to maintain homeostatic regulation of brain excitability (4,25,39). In rodents, the threshold dose for seizure onset induced by chemical convulsants (bicuculline, picrotoxin, pentylenetetrazol, and strychnine) changes over the estrus cycle. Female rats in estrus are more sensitive to chemical convulsants than are females in diestrus and males, whereas infusion of a progesterone metabolite increases the seizure threshold more for females in diestrus (25). The differential effects of estrogens on neuronal excitability also depend on cycling status. Excitability is enhanced when female rats in lowestrogen states are given estrogen (diestrus) but not when estrogen is given during a high-estrogen state (diestrus) (43).
Anatomic specificity in the cortical distribution of steroid hormone receptors may account for some of the differential effects of each steroid hormone on neuronal excitability, endocrine function, and reproductive behavior. Estrogen receptors are located primarily in the mesial temporal lobe (limbic cortex) and hypothalamus (3,44,45); progesterone and androgen receptors are also diffusely distributed over the cerebral cortex (40,45, 46, 47, 48, 49). Many of estrogen’s effects, including the steroid-dependent suppression of GAD, are confined to the CA1 region of the hippocampus (50). Anatomic distribution also varies with development. Neocortical receptors for estrogen in the immature brain are largely absent after puberty (3,51). Anatomic specificity and varying distribution might account, in part, for changes in seizure expression with changes in reproductive function.
EFFECTS OF SEIZURES ON REPRODUCTIVE HORMONES
Seizures themselves can alter the level of some hormones, particularly hypothalamic tropic hormones and pituitary gonadotropins (52), leading ultimately to changes in secretion of gonadal steroids.
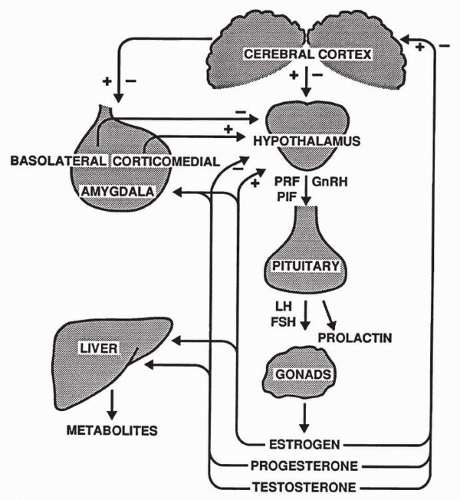
The hypothalamus regulates secretion of anterior pituitary hormones through the release of neurohormones, which in turn stimulate or inhibit release of hormones of the anterior pituitary. Gonadotropin-releasing hormone (GnRH), a hypothalamic tropic hormone, is released episodically and stimulates the pulsatile release of the pituitary gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH promotes development of the primary ovarian follicle and secretion of estradiol in the female, and spermatogenesis in the male (Fig. 48.1). In females, a midcycle surge of LH stimulates ovulation and formation of the progesterone-secreting corpus luteum. In males, LH stimulates interstitial cell secretion of testosterone and other androgens.
Pituitary release of prolactin is also determined by inhibitory and stimulating factors from the hypothalamus. Prolactin initiates milk synthesis in the mammary glands and affects growth, osmoregulation, and fat and carbohydrate metabolism. Prolactin also inhibits sexual behavior (53) and promotes parental behavior (54). Elevated levels can cause impotence in human males (55).
Steroid hormones are produced by the adrenal cortex and the gonads. The principal gonadal androgens are testosterone, dihydrotestosterone, and androstenedione. Dihydroandrostenedione is produced by the adrenal cortex. Testosterone is necessary for masculinization during sexual differentiation, spermatogenesis, and development of male secondary sexual characteristics at puberty, as well as for activation of sexual and aggressive behavior and maintenance of sexual desire.
The principal female sex hormones are estrogen and progesterone. The granulosa cells of the ovarian follicle produce estrogens, including estradiol, estrone and estriol, that support the development of female secondary sexual characteristics and also affect metabolic rate, body temperature, skin texture, and fat distribution. In addition, estrogens influence sexual and parental behavior. Progesterones are produced by the corpus luteum formed from the ovarian follicle after ovulation. Progesterone is essential for uterine, vaginal, and mammary gland growth. It inhibits the menstrual cycle to maintain pregnancy and has complex effects on sexual behavior.
HORMONE DISTURBANCES IN EPILEPSY
Among the hormonal abnormalities described in persons with epilepsy are changes in the release of LH, prolactin, and gonadal testosterone that may arise as a result of seizures or treatment with AEDs.
Seizures may disturb the hypothalamic-pituitary-gonadal axis, which is modulated by the cerebral and limbic cortex. Regions of limbic cortex, particularly the amygdala, have extensive reciprocal connections with the hypothalamus (56). In the amygdala, the corticomedial nuclear group stimulates hypothalamic release of GnRH, and the basolateral nuclear group inhibits its release (57), depending on which group is affected by excitation of the amygdala. The inhibitory or stimulatory effect ultimately alters release of the corresponding pituitary hormones (58), as does seizure-precipitated release of excitatory and inhibitory neurochemicals (21), which regulate neuroendocrine function.
In men and women with epilepsy, basal concentrations of LH and LH pulse frequency are abnormal (59, 60, 61, 62, 63), probably from derangement of the hypothalamic GnRH pulse generator (59). Women with epilepsy not treated with AEDs had a significant increase in gonadotropin basal secretion during frequent interictal epileptiform activity. Release of LH may vary according to the epilepsy syndrome and AED exposure. LH pulsatility increased in women with a variety of untreated epilepsies (63), but diminished in those taking AEDs for temporal lobe epilepsy (62). Baseline LH levels in men receiving AEDs may be lower than normal, with an exaggerated response to GnRH (64, 65, 66).
Growth hormone and prolactin are elevated interictally in some men and women with epilepsy (45,64,67,68). Pituitary prolactin increases more than twofold after generalized convulsive seizures, most complex partial seizures, and simple partial seizures involving limbic structures but not after nonepileptic seizures (69, 70, 71). The increase occurs within 5 minutes, is maximal by 15 minutes, and persists for 1 hour (72). Other changes include elevation in corticotropin and cortisol following both convulsions and stimulation of mesial temporal lobe structures.
Levels of sex steroid hormone may be abnormal in some men and women with epilepsy, in part because of changes in steroid metabolism induced by AEDs (73). AEDs that induce the hepatic cytochrome P450 (CYP) system increase the metabolism of gonadal and adrenal steroid hormones. AEDs also induce the synthesis of sex hormone-binding globulin (SHBG), a binding protein for steroid hormones (74). Increased protein binding decreases the free, biologically active fraction of hormone. Men who receive AEDs that induce the microsomal enzyme system may have low levels of total and free testosterone and of adrenal androgens (75, 76, 77, 78). Women taking such AEDs have low androgen and estrogen levels and elevated levels of SHBG protein (79,80). Valproate, which does not induce liver cytochrome enzymes, increases gonadal and adrenal androgen levels in women (81). Estrogen, progesterone, and androgen levels in women receiving gabapentin or lamotrigine, which do not affect on cytochrome enzymes, are not different from levels in medically normal nontreated
control women. These observations suggest that changes in steroid hormones are related to AED-associated alterations in steroid metabolism and not to seizures or epilepsy (79).
control women. These observations suggest that changes in steroid hormones are related to AED-associated alterations in steroid metabolism and not to seizures or epilepsy (79).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree