DHTN, diastolic hypertension; PHTN, prehypertension; SHTN, systolic hypertension; TF, Second Task Force Report (Task Force on Blood Pressure Control in Children, 1987); WG, Working Group Report (National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents, 1996).
The impact of the childhood obesity epidemic on the prevalence of hypertension in the young can be seen in several recent studies from the Houston Screening Project (McNiece et al., 2007a; Sorof et al., 2002, 2004a). In multiple publications, these investigators have demonstrated an increased prevalence of hypertension among obese children—as high as 4.5%—compared to nonobese children. Indeed, a recent examination of BP data in 8- to 17-year-old children from the NHANES and other related population-based studies conducted in the United States (U.S.) from 1963 to 2002 clearly demonstrates an increase in the prevalence of elevated BP in children (Fig. 16-1), with much of the increase attributable to the increase in childhood obesity (Din-Dzietham et al., 2007). According to this analysis, the prevalence of prehypertension has now reached 10% and the prevalence of hypertension nearly 4%. Of significant concern is that this increase has been much greater in non-Hispanic black and Mexican American children than in white children (Fig. 16-1).
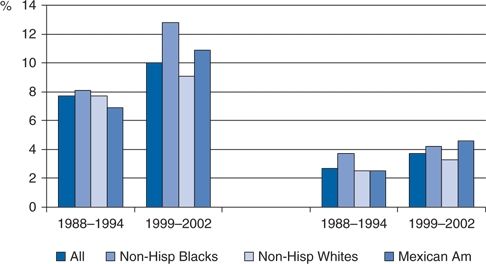
FIGURE 16-1 Prevalence of prehypertension (left-hand bars) and hypertension (right-hand bars) among American children in 1999 to 2002 compared to 1988 to 1994. (Data from Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 2007;116:1488–1496.)
Similar findings have been seen in screening studies performed in other countries, including China (Cao et al., 2012) and Iceland (Steinthorsdottir et al., 2011) (Table 16-1). Thus, it is apparent that the increased prevalence of high BP in children is a global phenomenon, likely related to the increasing prevalence of childhood obesity worldwide (Flynn, 2013).
CHILDHOOD PRECURSORS OF ADULT HYPERTENSION AND CARDIOVASCULAR DISEASE
It is increasingly clear that adult hypertension and other cardiovascular diseases have their origins in childhood. Not only BP levels but also other known cardiovascular risk factors can be measured in the young and then related to the subsequent development of hypertension and its cardiovascular manifestations in adult life (Expert Panel, 2011). The significance of hypertension in the young is further underscored by the many studies documenting the occurrence of hypertensive target organ damage in children and adolescents.
Blood Pressure Tracking
The pattern of BP over time, referred to as tracking, has been supported by data from a number of longitudinal cohort studies, most notably those conducted in Muscatine, Iowa (Lauer et al., 1993), and Bogalusa, Louisiana (Berenson, 2002). In all studies, the best predictive indicator of subsequently sustained elevated BP is an antecedent elevated BP level (Bao et al., 1995). Although an initially elevated BP level may not evolve into later sustained elevation, Lauer et al. (1993) found that 24% of young adults whose BP ever exceeded the 90th percentile as children had adult BP greater than the 90th percentile, a percentage that is 2.4 times higher than expected. In the Bogalusa cohort, 40% of those with systolic BP and 37% of those with diastolic BP above the 80th percentile at baseline continued to have BP above the 80th percentile 15 years later (Bao et al., 1995). More recently, data from the Fels Longitudinal study have added further weight to the concept that an elevated childhood BP reading predicts an increased chance of adult hypertension (Carrico et al., 2013).
Tracking is more consistent if the elevated childhood BP levels are combined with obesity, a parental history of hypertension, or increased left ventricular mass by echocardiography (Lauer et al., 1993; Shear et al., 1986). However, the strength of tracking appears to decrease with longer periods of follow-up (Chen & Wang 2008; Toschke et al., 2010).
In view of the higher prevalence of hypertension in black adults than in white adults, comparisons of the tracking phenomenon in black and white children have been made (Lane & Gill, 2004). Black children have significantly higher mean BP than white children even after adjustments for potential confounders such as weight gain (Bao et al., 1995), growth, or socioeconomic status (Dekkers et al., 2002). Dekkers et al. (2002) found that ethnic differences in systolic BP become manifest earlier in girls than in boys, and both systolic and diastolic differences tended to increase with age.
The importance of BP tracking was highlighted in a meta-analysis of 50 cohort studies conducted between 1970 and 2006 (Chen & Wang, 2008). The average tracking coefficient was 0.38 for systolic BP and 0.28 for diastolic BP, and the strength of BP tracking increased with baseline age for both systolic and diastolic BP. Similar modest tracking coefficients were found in another meta-analysis that was performed to examine the impact of BP tracking on longitudinal intervention trials (Toschke et al., 2010). Taken together, these data indicate that BP does track over time at a population level and support interventions designed to prevent future development of hypertension.
Hypertensive Target Organ Damage in the Young
Left ventricular hypertrophy (LVH), increased carotid intima media thickness (cIMT), and even impaired cognitive function stand as concrete evidence of the consequences of elevated BP in childhood and the potential for lifelong morbidity. LVH was first demonstrated to occur in hypertensive youth by Laird and Fixler (1981) and has subsequently been shown to occur in a significant proportion of hypertensive children and adolescents, with reported prevalences ranging between 20% and 41% depending upon the diagnostic criteria utilized (Brady et al., 2008; Flynn & Alderman, 2005; Hanevold et al., 2004; McNiece et al., 2007b; Sorof et al., 2003). The prevalence of LVH may be affected by ethnicity (Brady et al., 2010; Hanevold et al., 2004), concurrent obesity (Falkner et al., 2013; Hanevold et al., 2004), and the degree of BP elevation (Falkner et al., 2013; McNiece et al., 2007b). Only one study performed in hypertensive children has failed to demonstrate any relationship between LVH and specific parameters of BP elevation (Brady et al., 2008).
Increased cIMT, well documented as a cardiovascular consequence of elevated BP in large population studies (Vos et al., 2003), has also been found in children and adolescents with primary hypertension in single-center reports (Lande et al., 2006; Litwin et al., 2006; Sorof et al., 2003). While early studies of cIMT in hypertensive youth were confounded by the effects of obesity (Litwin et al., 2006; Sorof et al., 2003), one carefully conducted study that controlled for body mass index (BMI) demonstrated a definitive relationship between elevated BP itself and increased cIMT in young patients (Lande et al., 2006).
An additional target organ effect of elevated BP that has been described in the young is impaired cognitive function (Lande et al., 2003). While long-standing hypertension has long been recognized as a risk factor for the development of cognitive impairment and even dementia in the elderly (Paglieri et al., 2004), this study demonstrated that children and adolescents with BP greater than 90th percentile had poorer performance on selected tests of cognition compared to normotensive children. In a recent follow-up study, hypertensive children were found to have decreased executive function that was associated with decreased cerebrovascular reactivity in response to hypercapnia (Ostrovskaya et al., 2013). These provocative findings, while requiring confirmation, add impetus to consensus recommendations for instituting antihypertensive drug therapy in children and adolescents with persistently elevated BP.
Fewer pediatric data are available on the other major target organ effect of hypertension, namely renal damage. Although hypertension commonly accompanies chronic kidney disease (CKD) in children, it is rarely its cause (Shatat & Flynn, 2005). Even microalbuminuria, which is commonly seen in hypertensive adults, is infrequently seen in children with isolated hypertension, even when LVH is present (Sorof et al., 2004b). However, a more recent study demonstrated that approximately 58% of hypertensive adolescents had microalbuminuria, with an increased prevalence in stage 2 hypertension compared to stage 1 (Assadi, 2007). Reduction of BP in the latter study was accompanied by a reduction in both microalbuminuria and LVH. And another study showed that children with prehypertension on ambulatory BP monitoring (ABPM) had higher urinary protein excretion and lower glomerular filtration rate than normotensive children, albeit within the normal range (Lubrano et al., 2009). Thus, there may be increasing evidence that high BP even in childhood has detrimental renal effects.
Childhood BP and Subsequent CV Disease
As has been recently pointed out by the U.S. Preventive Services Task Force (Thompson et al., 2013), there are no data at present that clearly document a relationship between childhood BP and cardiovascular morbidity and mortality in adulthood. However, a number of studies have shown that BP and other traditional cardiovascular risk factors in childhood predict the subsequent presence of increased cIMT (Davis et al., 2001; Li et al., 2003; Raitakari et al., 2003; Vos et al., 2003) and increased arterial stiffness (Juonala et al., 2006; Li et al., 2004), two well-accepted surrogate markers for atherosclerosis.
Additionally, longitudinal studies have demonstrated that children with elevated BP are at increased risk of development of the metabolic syndrome as adults (Sun et al., 2007) and that components of the metabolic syndrome, an important risk factor for cardiovascular morbidity, track over time from childhood to adulthood (Chen et al., 2007). Taken together, these data indicate that over time, adult morbidity and mortality will be more tightly connected with childhood precursors and emphasize the need for early intervention (Expert Panel, 2011).
POTENTIAL CAUSATIVE FACTORS OF CHILDHOOD HYPERTENSION
Multiple factors have been reported to correlate with BP levels in children (Table 16-2) and have been examined as potential causative factors for childhood hypertension. Some factors are either genetic or environmental, but most have contributions of both. Height, body mass, and somatic development depend not only on genetic influences but also on nutrition and exercise.
TABLE 16-2 Factors Related to Blood Pressure Levels in Children and Adolescents
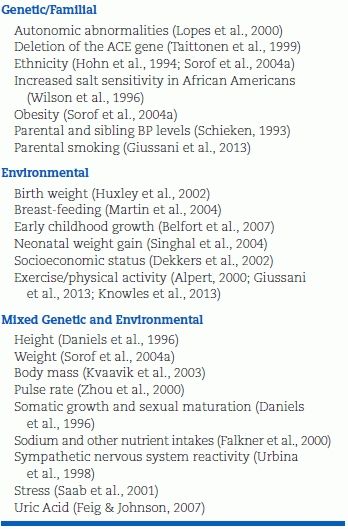
The Critical Role of Obesity
Obesity is growing at an alarming pace among children and adolescents in all developed societies, with—as in many other aberrant behaviors—the U.S. leading the way (Wang & Lobstein, 2006). Recent data indicate that this trend shows no signs of abating (Ogden et al., 2010); indeed, among younger children, the prevalence of obesity continues to increase worldwide (de Onis et al., 2010). Unfortunately, adolescent obesity tracts closely with adult obesity (Kvaavik et al., 2003), setting the foundation for all of the consequences. Interestingly, a recent meta-analysis indicates that childhood obesity alone is not an independent factor for adult cardiovascular disease, except among those whose BMI increases from childhood to adulthood, supporting the concept that intervention in childhood is crucial for prevention of adult cardiovascular disease (Lloyd et al., 2010).
Mainly as a consequence of increasing obesity, the mean BP of US children and adolescents has risen by 1.4/3.3 mm Hg from 1990 to 2000 (Muntner et al., 2004), and the prevalence of hypertension and prehypertension has increased (Din-Dzietham et al., 2007). The pathophysiologic links between childhood obesity and the development of hypertension, including the crucial role of sympathetic nervous system activation, have recently been reviewed (Flynn, 2013).
Low Birth Weight and Early Childhood Growth
Population studies conducted by Barker and others have demonstrated an inverse correlation between birth weight and adult BP (Gamborg et al., 2007; Law et al., 2002; Zureik et al., 1996). A relationship between birth weight and coronary heart disease and type 2 diabetes has also been noted (Barker et al., 2002). Proposed explanations for these findings include deficient maternal nutrition (Barker et al., 1993; Law et al., 1991), possibly leading to acquisition of a reduced number of nephrons (Mackenzie et al., 1996). Autopsy studies demonstrating a reduced number of nephrons in patients with primary hypertension (Keller et al., 2003) have added intriguing evidence to the latter hypothesis.
Other data indicate that early childhood growth may be more important than birth weight as an influence on future BP. Those children who were small at birth but who have accelerated weight gain either very early after birth (Singhal et al., 2003) or between ages 1 and 5 (Law et al., 2002) have more insulin resistance, obesity, and hypertension later in life. This association between rapid postnatal weight gain and higher BP has been prospectively documented in 3-year-olds (Belfort et al., 2007), 8-year-olds (Burke et al., 2004), and 11- to 14-year-olds (Falkner et al., 2004).
Those infants who are breast-fed and thereby have a lower rate of weight gain during infancy have lower BPs in later life than those who are fed enriched formula (Singhal et al., 2001). Although this protection against higher BP by breast-feeding may have been exaggerated by selective publication (Owen et al., 2003), the weight of evidence supports an association (Martin et al., 2004). Whether there is more to breast-feeding than a reduced rate of excess weight gain (Grummer-Strawn & Mei, 2004) is uncertain, but slower early growth appears to be beneficial for long-term cardiovascular health (Singhal et al., 2004).
Genetic Factors
The hereditability of BP was established decades ago by the findings of a correlation of BP levels between parents and their natural offspring but no correlation between parents and their adopted children (Biron et al., 1976). Recently published studies have demonstrated that a large percentage of children and adolescents with primary hypertension have positive family histories of hypertension in a parent or grandparent (Flynn & Alderman, 2005; Robinson et al., 2005). Genetic influences on BP have been shown in comparisons of siblings (Wang et al., 1999) and twins (Kupper et al., 2006).
Table 16-3 lists some of the differences reported among normotensive children with a positive family history versus those with a negative family history of hypertension. It is likely that yet-undiscovered genetic polymorphisms may account for the development of “primary” hypertension in families and that these in combination with environmental factors may explain the early appearance of hypertension in some nonobese children and adolescents.
TABLE 16-3 Characteristics of FH+ Normotensive Compared with FH– Normotensives

SBP, systolic blood pressure.
Environmental Factors
Of environmental factors that can affect BP, increased body mass has already been discussed as a major determinant of higher BP levels throughout childhood and adolescence. The relationship between sodium and BP is comprehensively reviewed in Chapter 6. A recent analysis of childhood sodium intake and BP conducted in Great Britain showed that an increase of 1 g/day in salt intake was related to an increase of 0.4 mm Hg in systolic BP (He et al., 2008). Sodium intake may exert its effect on BP in those who are genetically predisposed to higher BP levels and are sodium sensitive, especially African Americans (Wilson et al., 1996). Obese adolescents also have heightened responsiveness to sodium intake (Rocchini et al., 1989).
Other dietary constituents, including calcium, potassium, protein and fiber, have been shown in an intervention study to have inverse associations with BP in children and adolescents (Simons-Morton et al., 1997). Increased intake of dairy products in 8-year-old children leads to higher intake of calcium, potassium, and magnesium, which was accompanied by lower systolic and diastolic BP (Rangan et al., 2012). In another study, Falkner et al. (2000), using folate as a surrogate for adequacy of micronutrient intake, concluded that African American adolescents with higher folate and micronutrient intakes had lower mean diastolic BP. Caffeine intake has also been associated with an elevated BP in adolescents, with the effect greater in African Americans than in Caucasians (Savoca et al., 2004).
PREVENTION OF HYPERTENSION
The need for early recognition and appropriate management of elevated BP in children is being increasingly emphasized (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004). Practitioners working with children and their families are in an ideal position to introduce preventive measures that will ensure future cardiovascular health (Expert Panel, 2011; Kavey et al., 2003).
Children and their families need detailed information about optimal dietary intakes, with appropriate cultural orientation. Once the dietary needs for cholesterol and myelinization of the central nervous system have been met (typically by the age of 2 years), recommendations for a prudent intake of fat such as in the DASH diet (Appel et al., 1997; Couch et al., 2008) should be provided. Family meals are an ideal setting to create lifetime healthful food habits.
Similarly, family activities that include age-appropriate exercise are helpful, not only to prevent hypertension but also to control obesity (Torrance et al., 2007). Families must be informed of the deleterious effects of pressor agents—including tobacco, street drugs, and nonsteroidal anti-inflammatory drugs—and their potential to increase BP with chronic use. With these proactive steps, the health of children will be improved. Whether adult hypertension will be prevented remains unknown.
CLASSIFICATION AND DIAGNOSIS OF HYPERTENSION IN CHILDREN AND ADOLESCENTS
Diagnostic criteria for elevated BP in childhood are based on the concept that BP in children increases with age and with body size, which makes it impossible to utilize a single BP level to define hypertension as is done in adults. This was recognized by early investigators of juvenile hypertension, who initially adopted the adult threshold of 140/90 but later realized that this represented a severe level of BP elevation in pediatrics, particularly in young children, and that population data were needed in order to better define the normal BP distribution and what constitutes an elevated BP in the young (Loggie, 1977).
Under the auspices of the National Heart, Lung and Blood Institute, consensus guidelines with recommendations for identification and management of elevated BP in childhood have been issued on four occasions since 1977. These guidelines have also included normative data on childhood BP derived from large-scale, cross-sectional studies of BP in healthy children. The most recent of these guidelines “The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents” (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004) adapted terminology and staging criteria utilized in the JNC-7 consensus guidelines for adult hypertension (Chobanian et al., 2003) to the problem of childhood hypertension and emphasized the prevention of adult cardiovascular disease by early intervention in children and adolescents with elevated BP.
Since the publication of the Fourth Report, two additional sets of guidelines for childhood hypertension have been issued (Expert Panel, 2011; Lurbe et al., 2009), but with one notable exception (see discussion of treatment), neither of these documents differ substantially from the Fourth Report. Given the increase in knowledge about high BP in the young in the decade since publication of the Fourth Report, a revised pediatric guideline is clearly needed.
Definitions and Classification of Elevated Blood Pressure
As noted above, the definitions of normal and elevated BP in children aged 1 to 17 years are statistical constructs based upon the distribution of childhood BP:
- Normal BP: systolic and diastolic BP less than 90th percentile for age, gender and height (Tables 16-4 and 16-5)
- Prehypertension: systolic or diastolic BP between the 90th and 95th percentiles, or BP ≥120/80 in an adolescent
- Hypertension: systolic and/or diastolic BP persistently ≥95th percentile
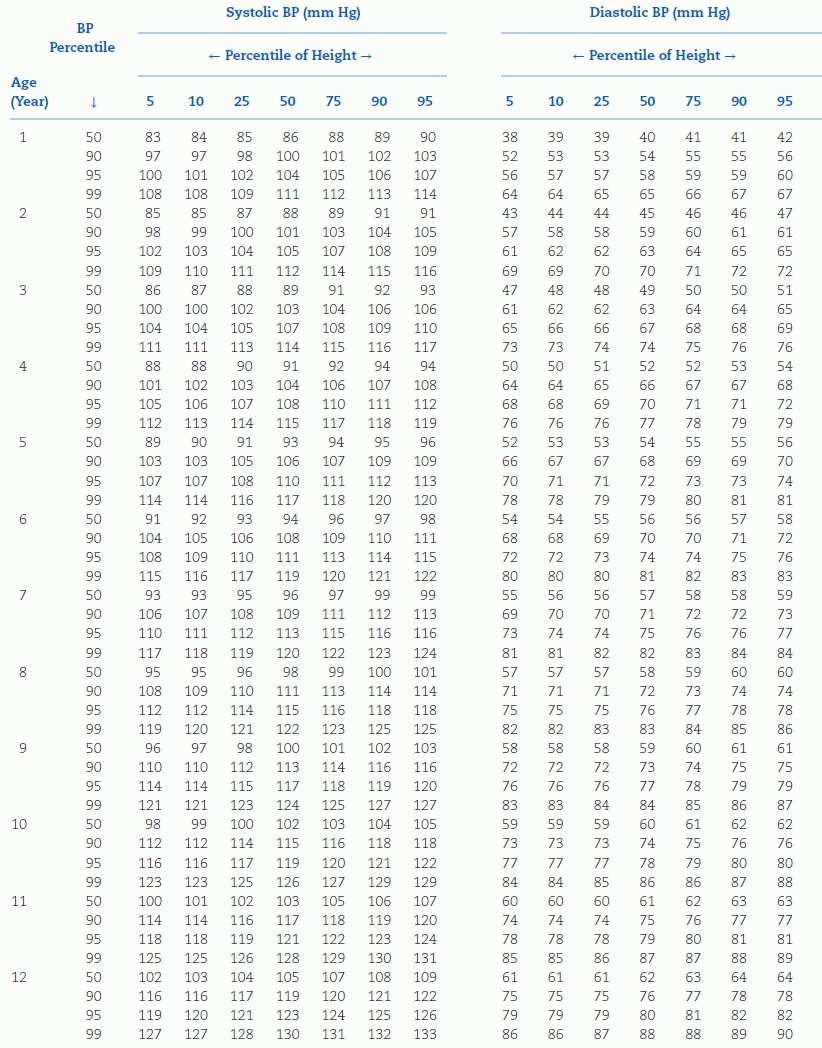
TABLE 16-4 Blood Pressure Levels for Boys by Age and Height Percentilea
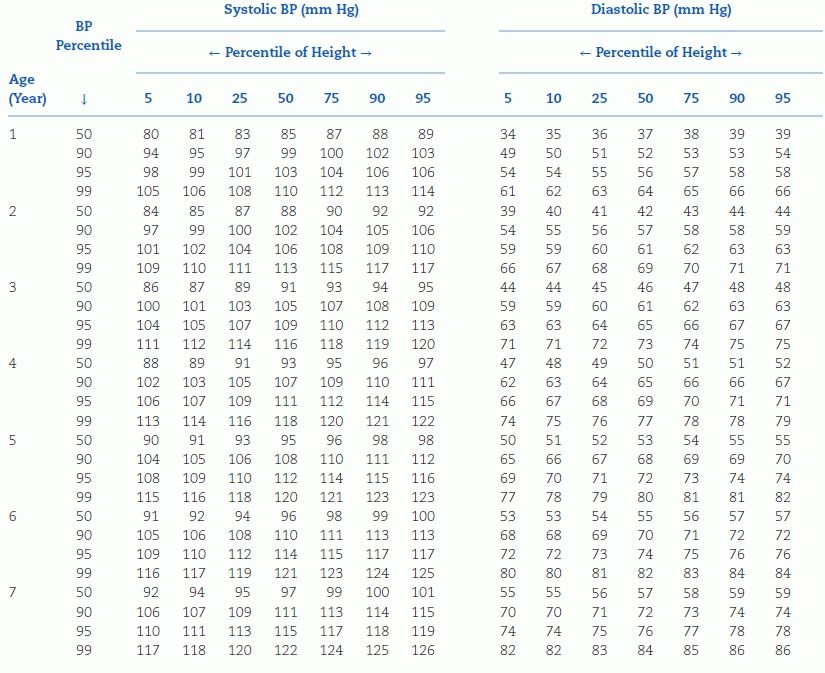
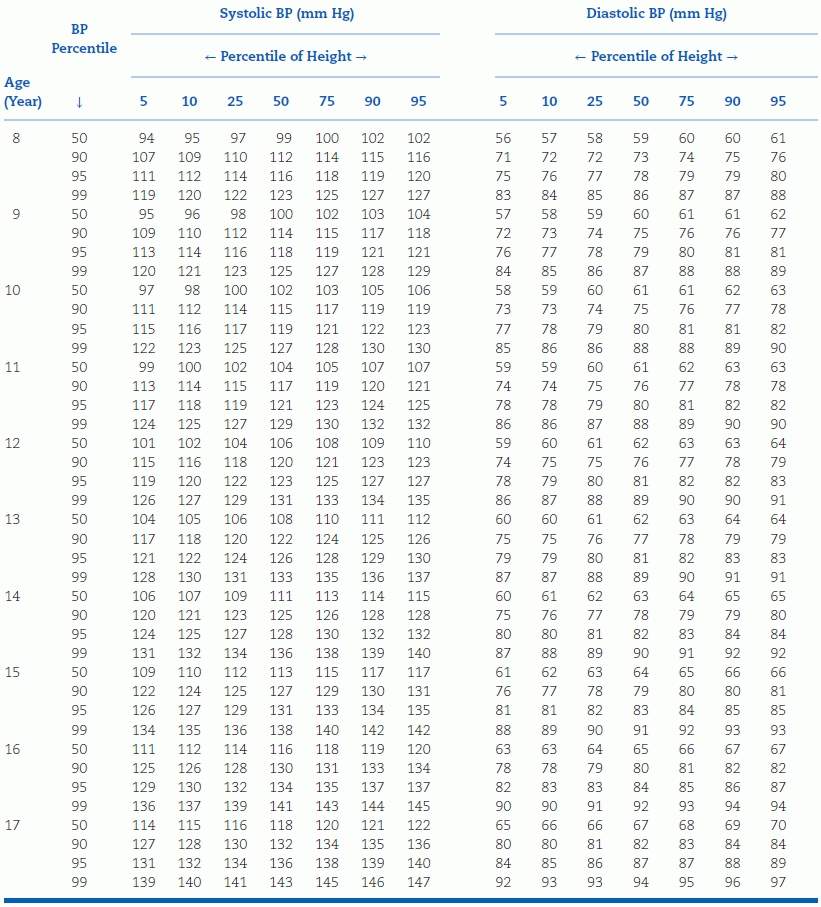
aTo use the table, first plot the child’s height on a standard growth curve (www.cdc.gov/growthcharts). The child’s measured SBP and DBP are compared with the numbers provided in the table according to the child’s age and height percentile.
BP, blood pressure. Reproduced from National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 2004;114:555–576.
TABLE 16-5 Blood Pressure Levels for Girls by Age and Height Percentilea
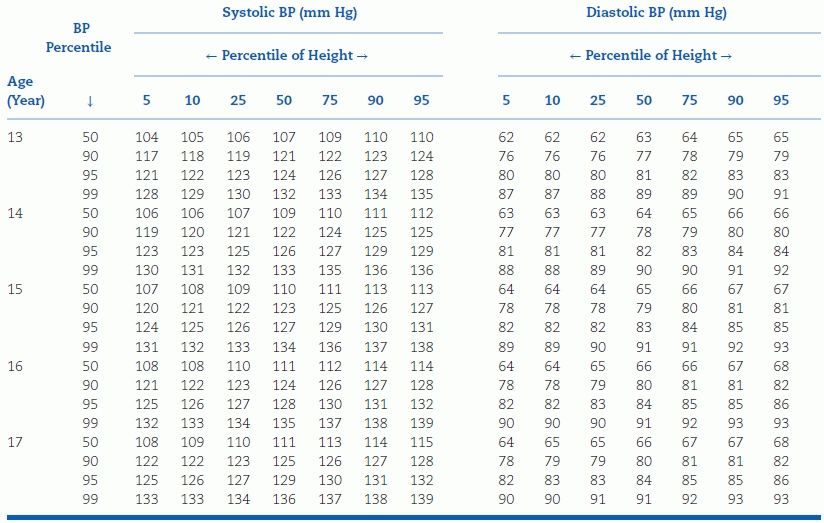
aTo use the table, first plot the child’s height on a standard growth curve (www.cdc.gov/growthcharts). The child’s measured SBP and DBP are compared with the numbers provided in the table according to the child’s age and height percentile.
BP, blood pressure. Reproduced from National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 2004;114:555–576.
While it would be preferable to have a risk-based definition of hypertension in the young (Chiolero, 2014), the above statistical definitions remain the only ones available at present.
The Fourth Report additionally provides guidelines for staging the severity of hypertension in children and adolescents, which can then be used clinically to guide evaluation and management (Table 16-6). Children or adolescents with stage 2 hypertension should be evaluated and treated more quickly and/or aggressively than those with lower degrees of BP elevation. The overall approach to the classification of elevated BP in children and adolescents is summarized in Figure 16-2.
TABLE 16-6 Classification of Hypertension in Children and Adolescents, with Measurement Frequency and Therapy Recommendations
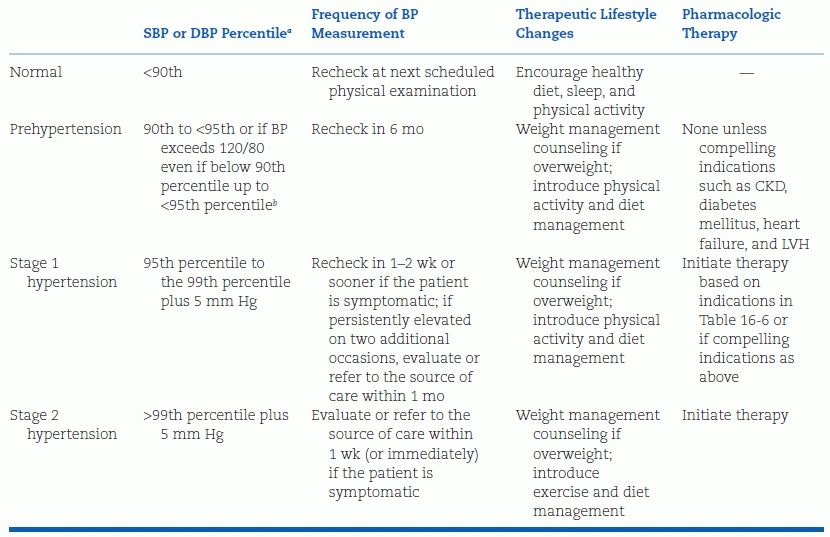
aFor sex, age, and height measured on at least three separate occasions; if systolic and diastolic categories are different, categorize by the higher value.
bThis occurs typically at 12 years old for SBP and at 16 years old for DBP.
BP, blood pressure; CKD, chronic kidney disease; DBP, diastolic blood pressure; LVH, left ventricular hypertrophy; SBP, systolic blood pressure. Adapted from National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 2004;114:555–576.
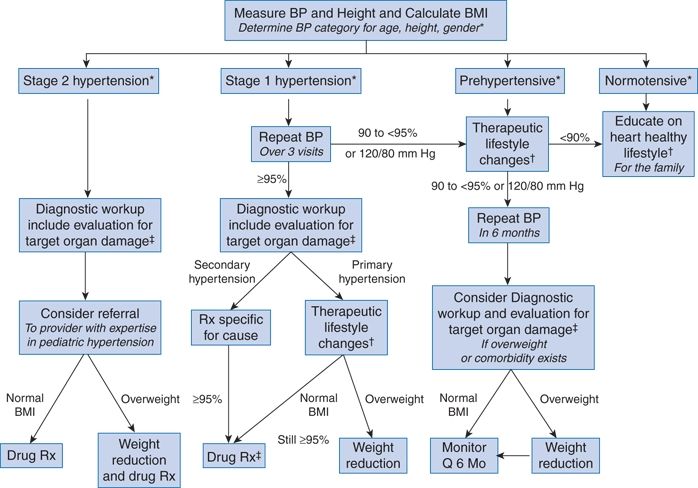
FIGURE 16-2 Suggested management algorithm for children and adolescents with elevated blood pressure. BMI, body mass index; Q, every. *See Tables 16-4–16-6. †Diet modification and physical activity. ‡Especially if younger, very high BP, little or no family history, diabetic, or other risk factors. (Reproduced from National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 2004;114:555–576.)
Assessment
Confirmation of BP Elevation
The first step in evaluating the hypertensive child or adolescent is to confirm that the BP is truly elevated. Since the BP distributions published in the Fourth Report are based upon auscultated BPs, and given the inherent inaccuracies of oscillometric BPs and their variation from auscultated BPs in the young (Butani & Morgenstern, 2003; Eliasdottir et al., 2013; Park et al., 2001), it is recommended that if a child’s BP is found to be elevated using an automated device, it should be confirmed by auscultation. Exceptions to this would include infants and young children who are unable to cooperate with manual BP determination. Furthermore, unless there are symptoms of hypertension present, the child’s or adolescent’s BP should be shown to be elevated on at least three occasions before making the diagnosis of hypertension (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004).
Techniques for manual BP measurement recommended by the American Heart Association (Pickering et al., 2005) with respect to cuff size, patient position, etc. should be followed in children and adolescents whenever feasible. As in adults, the fifth Korotkoff sound should be reported as the diastolic BP, except in those children and adolescents in whom Korotkoff sounds can be heard down to “zero”; in such children, the fourth Korotkoff sound should be reported as the diastolic BP (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004).
Ambulatory BP Monitoring, White-Coat and Masked Hypertension
ABPM has been endorsed as an appropriate technique for the evaluation of elevated BP in children and adolescents (Flynn et al., 2014; National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004; Urbina et al., 2008). Recommended indications for performance of ABPM in children include identification of white-coat and masked hypertension, assessment of BP control in those treated with antihypertensive medications, and investigation of hypotensive episodes (Flynn & Urbina, 2012; Flynn et al., 2014; Lurbe et al., 2004; Urbina et al., 2008). The use of ABPM in a referred pediatric population has been shown to reduce the cost of evaluation of elevated BP by identifying those with white-coat hypertension, who then could receive a less-extensive workup (Swartz et al., 2008).
Children and adolescents with secondary hypertension have been found to have more significant nocturnal hypertension and greater daytime diastolic hypertension than those with primary hypertension (Flynn, 2002) as well as blunted nocturnal dipping (Seeman et al., 2005), suggesting that ambulatory monitoring can be used to identify children who need a more exhaustive evaluation for underlying causes of secondary hypertension.
White-coat hypertension appears to be at least as common in children as it is in adults (Flynn & Urbina, 2012), although it should be noted that white-coat hypertension is less likely at higher levels of office BP (Sorof et al., 2001). Several studies have indicated that children found to have white-coat hypertension actually have early signs of target organ damage, such as increased left ventricular mass (Kavey et al., 2007; Lande et al., 2008; Stabouli et al., 2005). These data, in combination with the data from tracking studies that suggest that these children are likely at increased risk of development of hypertension in the future, imply that children found to have white-coat hypertension should receive lifestyle modification and should be followed prospectively for the development of definite hypertension.
Masked hypertension has also been recently described in pediatric populations (Lurbe et al., 2005; Matsuoka & Awazu, 2004; Stabouli et al., 2005) and is associated with hypertensive target organ damage, specifically LVH (Lurbe et al., 2005; McNiece et al., 2007b; Stabouli et al., 2005; Urbina, 2008). Such children probably merit further evaluation for underlying causes of hypertension and institution of pharmacologic treatment. However, since ABPM remains a specialized technique in pediatrics, further studies are needed to identify groups of children at increased risk of masked hypertension who could benefit from ABPM.
Differential Diagnosis
Traditionally, most hypertension in children has been felt to be secondary to an underlying disorder. As can be seen in Table 16-7, this is certainly the case for infants and young children. In hypertensive children in these age groups, renal disease, renovascular disease, and cardiac disease will often be found after an appropriate diagnostic evaluation. This was illustrated quite clearly in a recently published analysis of subjects enrolled in two antihypertensive drug studies: 80% of enrolled children less than 6 years of age had secondary causes of hypertension (Flynn et al., 2012). Primary hypertension in young children is therefore usually considered a diagnosis of exclusion.
TABLE 16-7 Causes of Childhood Hypertension by Age Group
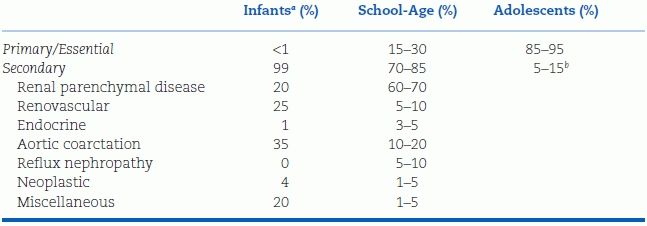
aLess than 1 y of age.
bBreakdown of causes is generally similar to that for school-age children.
In adolescents, however, hypertension is most likely to be primary in origin. This was demonstrated two decades ago in a study of over 1,000 hypertensive children evaluated at a Polish children’s hospital (Wyszynska et al., 1992). In this series, the vast majority of adolescents with persistent BP elevation had no identifiable underlying cause found. Other features that support the diagnosis of primary hypertension include normal growth (and/or obesity), lack of symptoms of hypertension, unremarkable past medical history, and a family history of hypertension (Flynn & Alderman, 2005). Hypertensive adolescents that fit this profile may not need as extensive an evaluation as those who do not.
Diagnostic Evaluation
Hypertension in childhood and adolescence is typically asymptomatic, although up to half of patients may report one or more symptoms, most frequently headache (Croix & Feig, 2006). In adolescent athletes, headaches may occur after strenuous exercise. Symptoms such as seizures, nosebleeds, dizziness, and syncope are rare and, if present, suggest that the BP elevation has been exacerbated by ingested substances or by emotional upset. On the other hand, if these symptoms occur in conjunction with elevated BP in a younger child, they may be a clue to the presence of secondary hypertension. For this reason, it is important to include a systems review designed to elicit signs and symptoms of underlying conditions such as renal disease that may be causing the elevated BP.
The family history should include not only hypertension but also associated conditions and complications such as dyslipidemia, stroke, myocardial infarction, and diabetes. Many substances commonly used or abused in children and adolescents can elevate BP, including prescribed and over-the-counter medications (e.g., corticosteroids or decongestants) and street drugs such as amphetamines and cocaine.
The physical examination should begin with plotting of growth parameters, especially height and BMI, and measurement of BP in both arms and at least one leg. From there, the examination should be focused on detecting the signs of secondary causes of hypertension, such as decreased femoral pulses, abdominal bruits, and cushingoid stigmata (Table 16-8).
TABLE 16-8 Physical Examination Findings and Etiology of Hypertension in Children and Adolescents
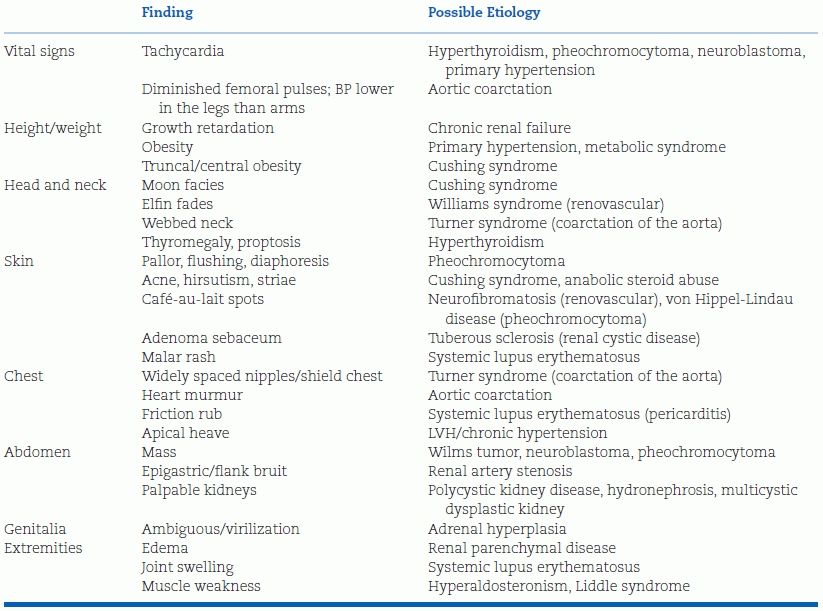
Except in young children, the likelihood that an asymptomatic child with persistently elevated BP will have an underlying cause for the elevation is remote low. In children with an identifiable cause for their hypertension, the history and physical examination usually reveal suggestive evidence of the cause, so detailed diagnostic evaluation of children without suggestive evidence is not warranted. Basic screening tests, including serum chemistries and lipids as well as a urinalysis, should be obtained in all patients. Specific specialized studies may be required in some children, particularly those with symptomatic hypertension or stage 2 hypertension (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004; Varda & Gregoric, 2005).
As discussed above, consideration should be given to including ABPM in evaluation of all children and adolescents with persistent office BP elevation (Flynn & Urbina, 2012), in order to identify children with white-coat hypertension and also to identify those with possible secondary hypertension. Given the high frequency of LVH in hypertensive children and adolescents (Flynn & Alderman, 2005; Hanevold et al., 2004; Sorof et al., 2004b), echocardiography should be considered part of the baseline evaluation, especially if pharmacologic intervention is required, so that reversal of abnormalities can be monitored and correlated with the adequacy of BP control.
MANAGEMENT OF HYPERTENSION IN CHILDREN AND ADOLESCENTS
Treatment of hypertension in children and adolescents is still largely empiric because there are no long-term studies of either dietary intervention or drug therapy. Even though more data are now available on safety and effectiveness of drug therapy than in the past (Ferguson & Flynn, 2013), the decision as to whether a specific child should receive medication must be individualized.
Nonpharmacologic Management
Guideline-issuing organizations emphasize that treatment of hypertension in children and adolescents should begin with nonpharmacologic measures (Fig. 16-2) (Expert Panel, 2011; National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004). Although the magnitude of change in BP may be modest, weight loss, aerobic exercise, and dietary modifications have been shown to reduce BP in children and adolescents (Bianchini et al., 2013; Maggio et al, 2011; Torrance et al., 2007). For example, for exercise, sustained training over 3 to 6 months has been shown to result in a reduction of 6 to 12 mm Hg for systolic BP and 3 to 5 mm Hg for diastolic BP (Alpert, 2000). Exercise has been shown to improve body composition and reduce other cardiovascular risk factors associated with obesity (Zorba et al., 2011). However, cessation of training is generally promptly followed by a rise in BP to preexercise levels. It is important to emphasize that aerobic exercise activities such as running, walking, and cycling are preferred to static forms of exercise in the management of hypertension.
Many children may already be participating in one or more appropriate activities and may only need to increase the frequency and intensity of these activities to see a benefit in terms of lower BP. Along the same lines, it is important to note that baseline physical activity has been shown to be inversely related to BP in children as young as 5 to 7 years (Knowles et al., 2013). Hypertension is not considered a contraindication to participation in competitive sports, so long as the child’s BP is “controlled” (McCambridge et al., 2010).
Several studies have demonstrated that weight loss in obese children and adolescents lowers BP (Hobkirk et al., 2012; Torrance et al., 2007). Weight loss not only decreases BP but also improves other cardiovascular risk factors such as dyslipidemia and insulin resistance (Bianchini et al., 2013; Reinehr et al., 2006). In studies where a reduction in BMI of about 10% was achieved, short-term reductions in BP were in the range of 8 to 12 mm Hg. Unfortunately, weight loss is notoriously difficult and usually unsuccessful, especially in the primary care setting. Comprehensive programs have better success rates. However, identifying a complication of obesity such as hypertension can perhaps provide the necessary motivation for patients and families to make the appropriate lifestyle changes.
The role of diet in the treatment of hypertension has received a great deal of attention, most of which has focused on sodium. Once hypertension has been established, “salt sensitivity” becomes more common, and reduction in sodium intake may be of benefit (Cutler, 1999; Hanevold, 2013). Other dietary constituents that have been examined in patients with hypertension include potassium and calcium, both of which have been shown to have antihypertensive effects (Cutler, 1999; Mu et al., 2005). Therefore, a diet that is low in sodium and enriched in potassium and calcium may be even more effective than a diet that restricts sodium only.
An example of such a diet is the so-called “DASH” diet, which has been shown to have a clear BP-lowering effect in adults with hypertension, even in those receiving antihypertensive medication (Appel et al., 1997). A study of a DASH-type eating plan confirmed its efficacy in lowering the BP in hypertensive children (Couch et al., 2008). The DASH diet also incorporates measures designed to reduce dietary fat intake, an important strategy given the frequent presence of both hypertension and elevated lipids in children and adolescents and the imperative to begin prevention of adult cardiovascular disease at as early an age as possible (Expert Panel, 2011; Kavey et al., 2003).
Pharmacologic Management
As discussed earlier, ample data exist documenting the development of hypertensive target organ damage in hypertensive children and adolescents, and a growing body of data suggests that elevated BP in the young may have adverse cardiovascular effects in adulthood. However, it has also been argued that the long-term consequences of untreated hypertension in an asymptomatic, hypertensive child or adolescent without underlying secondary hypertension or hypertensive target organ damage are completely unknown (Thompson et al., 2013). There is also a significant lack of data on the long-term effects of antihypertensive medications on the growth and development of children. Therefore, a definite indication for initiating pharmacologic therapy should be ascertained before medication is prescribed.
Accepted indications for use of antihypertensive medications in children and adolescents include the following (Lurbe et al., 2009; National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004):
- Symptomatic hypertension
- Secondary hypertension
- Hypertensive target organ damage
- Diabetes (types 1 and 2)
- Persistent hypertension despite nonpharmacologic measures (Fig. 16-2)
- Stage 2 hypertension
Pharmacologic reduction of BP for hypertensive children who fall into one of these categories is felt to result in health benefit.
The number of antihypertensive medications that have been systematically studied in children has increased markedly over the past decade due to incentives provided to the pharmaceutical industry under the auspices of the 1997 Food and Drug Administration Modernization Act (FDAMA) and subsequent legislation (Ferguson & Flynn, 2013; Flynn, 2003; Welch et al., 2012). Published results of the industry-sponsored clinical trials (which have been summarized elsewhere [Ferguson & Flynn, 2013]) can be used to guide the prescribing of antihypertensive agents in children and adolescents who require pharmacologic treatment, thereby increasing the confidence of the practitioner who treats such children. The dosing recommendations contained in Table 16-9 incorporate data from many of these studies.
TABLE 16-9 Recommended Doses for Selected Antihypertensive Agents for Use in Hypertensive Children and Adolescents
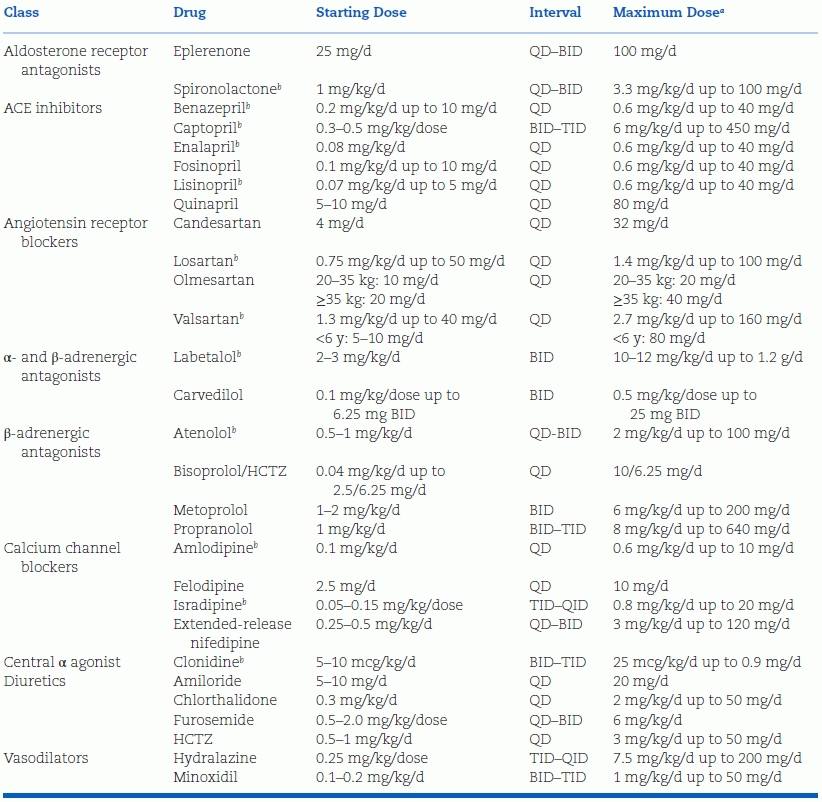
aThe maximum recommended adult dose should never be exceeded.
bInformation on preparation of a stable extemporaneous suspension is available for these agents.
BID, twice daily; HCTZ, hydrochlorothiazide; QD, once daily; QID, four times daily; TID, three times daily; ACE, angiotensin-converting enzyme.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








