Chapter 86 Idiopathic Hypersomnia
History
The term idiopathic hypersomnia was used as early as 1829 (“die idiopathische chronische Schlafsucht”) for excessive daytime sleepiness of undetermined origin.1 Bedrich Roth was the first in the late 1950s to describe a syndrome that was characterized by excessive daytime sleepiness, prolonged sleep, and sleep drunkenness and by the absence of sleep attacks, cataplexy, sleep paralysis, and hallucinations. The of independent sleep drunkenness and hypersomnia with sleep drunkenness were initially suggested.2–5 Overlapping features with narcolepsy were identified from the beginning and led to the use of such labels as essential narcolepsy, independent narcolepsy, and non–rapid eye movement (NREM) sleep narcolepsy.6,7 Other terms including idiopathic central nervous system hypersomnolence, functional hypersomnia, hypersomnia with automatic behavior, harmonious hypersomnia, and idiopathic hypersomnia (in the 1990 edition of the International Classification of Sleep Disorders [ICSD]) were also put forward. The 2005 version of the ICSD suggests differentiating between idiopathic hypersomnia with long sleep time (the polysymptomatic classic form of idiopathic hypersomnia) and idiopathic hypersomnia without long sleep time (the monosymptomatic form).
Epidemiology
Idiopathic hypersomnia is rare, and its incidence is usually overestimated. In the absence of systematic studies, the exact prevalence of idiopathic hypersomnia is unknown. Reports suggest that patients with idiopathic hypersomnia represent only about 1% of patients seen in neurologic sleep centers and are 5 to 10 times less common than patients with narcolepsy.8,9 Hence, the prevalence of idiopathic hypersomnia in the general population may be estimated at around 50 per million, a figure much lower than the 300 to 600 per million suggested until the early 1980s.4,5,10 Only about one fourth of patients with idiopathic hypersomnia diagnosed according to ICSD criteria present the classic or polysymptomatic form.8 In a large series published in 2007, patients with idiopathic hypersomnia represented 1% of 6000 patients seen at a single respiratory sleep center. Because idiopathic hypersomnia is 60% as prevalent as narcolepsy, questions about the diagnostic accuracy arise.11
The age of onset of symptoms varies, but it is commonly between 10 and 30 years. In contrast to narcolepsy with cataplexy, the age of onset is sometimes difficult to pinpoint due to the insidious onset of the condition over several weeks or months. Once established, symptoms are generally stable and long lasting. Spontaneous improvement in excessive daytime sleepiness may be observed in up to one quarter of patients.8,11,12 A female preponderance was found in some but not all series.8,11–13 In one to two thirds of cases (see later), idiopathic hypersomnia is familial.
Pathogenesis
Genetic and Environmental Factors
The disorder is familial in 50% to 60% of cases, most commonly in the classic (polysymptomatic) form. On rare occasions, idiopathic hypersomnia and narcolepsy occur in the same family.8,9,14 An autosomal dominant mode of inheritance has been discussed, and females may be more affected.13 In a few series, an association with diabetes or obesity was observed.8,15
Given the existence of overlapping features between idiopathic hypersomnia and narcolepsy (see later), there has been an interest in potential human leukocyte antigen (HLA) markers for idiopathic hypersomnia. Despite reports of an increase in HLA DQ1,8 DR5 and Cw2,16 and DQ317 and of a decrease of Cw3,18 no consistent findings have emerged. HLA typing currently does not play a role in the diagnosis of idiopathic hypersomnia.
Serum total IgG levels were recently reported in 159 Japanese with narcolepsy-cataplexy patients positive for the HLA-DQB1*0602 allele, 28 idiopathic hypersomnia patients with long sleep time, and 123 healthy controls (HLA-DQB1*0602 present in 45 of them). The distribution of serum IgG was significantly different among healthy controls (11.66 ± 3.55 mg/ml and 11.45 ± 3.43 for HLA-positive and HLA-negative subjects, respectively), narcolepsy patients (9.67 ± 3.38), and idiopathic hypersomnia patients (13.81 ± 3.80). In addition, idiopathic hypersomnia patients showed high IgG3 and IgG4 level, low IgG2 level, and IgG1/IgG2 imbalance.18a
Hypersomnia usually starts insidiously. Occasionally, excessive daytime sleepiness is first experienced after transient insomnia, abrupt changes in sleep–wake habits, overexertion, general anesthesia, viral illness or mild head trauma.8 Cerebrospinal fluid (CSF) analyses in idiopathic hypersomnia have shown normal cell counts, cytology, and protein content.
Neurochemistry
Montplaisir and coworkers found a decrease in dopamine and indole-3-acetic acid in both idiopathic hypersomnia and narcolepsy.19 Faull and colleagues found similar mean concentrations of monoamine metabolites in subjects with narcolepsy or idiopathic hypersomnia and with controls but—using a principal component analysis—they also found a dysregulation of the dopamine system in narcolepsy and dysregulation of the norepinephrine system in idiopathic hypersomnia.20–22
These metabolic data suggest the possibility of a dysfunction of aminergic arousal systems in idiopathic hypersomnia. Additional experimental and human data give some support to this hypothesis. In the cat, both hypersomnia and disturbances of monoamine metabolites can be induced by a lesion of ascending noradrenergic pathways.23 In humans, decreased CSF levels of histamine were found in patients with excessive daytime sleepiness including narcolepsy and idiopathic hypersomnia.24,25 Finally, the hypothesis of a dysfunctional dopaminergic transmission has been confirmed also by positron emission tomography (PET) studies.26
Most studies found normal levels of CSF hypocretin-1 in idiopathic hypersomnia.15,27–29 In the absence of clear cutoffs for normal levels and considering differences in diagnostic criteria, reports of diminished levels of hypocretin-1 and hypocretin-2 levels must be interpreted with caution.30,31
Neurophysiology
Homeostatic and circadian disturbances of sleep regulation as well as deficient arousal systems have been postulated in idiopathic hypersomnia. An abnormally high level of slow-wave activity (SWA), due either to an abnormally slow decay of SWA or to a normal decay of an enhanced level of SWA, was found by some authors.32,33 More-recent studies did not confirm these data. Reports of increased sleep spindle activity at the beginning and at the end of sleep and of a delayed start (and decline) of melatonin and cortisol secretion suggest a primary circadian deficit in idiopathic hypersomnia.34 Unfortunately, core temperature recordings have not been reported in idiopathic hypersomnia to date.
The detection after awakening of delayed and smaller P300 potentials in idiopathic hypersomnia documents a cortical activation problem in these patients without, however, giving any clue about its origin.33,35
Clinical Features
Excessive Daytime Sleepiness
A few patients with idiopathic hypersomnia may occasionally report episodes of irresistible sleep episodes as well as short and refreshing naps.8 The existence, on the other hand, of patients who have narcolepsy with cataplexy with nonimperative excessive daytime sleepiness, prolonged naps, and prolonged nocturnal sleep proved the existence of an overlap between narcolepsy and idiopathic hypersomnia, as suggested also by the occurrence of both conditions in the same family (see earlier).
In a comparison of 62 patients with idiopathic hypersomnia and 50 controls, patients were able to focus only for 1 hour (versus 4 hours in the controls) and felt more sedated in darkness, in a quiet environment, and when listening to music or conversation. Patients were also more frequently evening type and more alert in the evening than in the morning.35a
Nocturnal Sleep
Nocturnal sleep often is typically subjectively long and undisturbed, with more than 10 hours of sleep. A few patients report sleep times of 12 to 19 hours per day on weekends and during holidays. Because most patients do not report improvement of excessive daytime sleepiness with prolonged sleep, history of extreme sleep times (sleep longer than 12 hours per day) is uncommon.8 In a comparison of 62 patients with idiopathic hypersomnia and 50 controls, patients slept 3 hours more on weekends, on holidays, and in the sleep unit than on working days.35a
Awakening after nocturnal sleep is typically difficult. Sleep drunkenness (also called syndrome d’Elpénor, after the youngest of Ulysses’ comrades, who killed himself during an episode of incomplete awakening) is reported by 40% to 60% of patients,5,8,11 but can be seen also in other forms of excessive daytime sleepiness. Patients are hard to awaken; they can be aggressive and verbally and physically abusive during that twilight state if they are awakened, even at their own request. The patient may be confused and unable to react adequately to external stimuli upon awakening. The sleep inertia (time to get going) in the morning may be as long as 2 to 3 hours. Sleep drunkenness can also be noted when patients are awakened from naps.
Associated Features
Sleep paralysis and hallucinations are typical for narcolepsy with cataplexy, but they are not exceptional in patients with idiopathic hypersomnia.8,29,36 Cataplexy-like episodes and nightmares are also possible. Depressive symptoms were noted in 15% to 25% of patients in Roth’s series and confirmed in later studies.5,8,14,37,38 Mood changes not qualifying for the diagnosis of affective disorder can precede or follow the onset of excessive daytime sleepiness and evolve independently. The presence of major depression signs are, however, not compatible with the diagnosis of idiopathic hypersomnia (see later).
Headache is a common symptom in patients with excessive daytime sleepiness. Migraine-type and tension-type headaches are reported in about 30% of patients with idiopathic hypersomnia. Pain complaints of other localizations are occasionally observed. Neurovegetative symptoms such as cold hands or feet, lightheadedness on standing up (orthostatic hypotension), or syncope have been observed.3,8,12,39,40 The incidence of neurovegetative symptoms is, however, similar in narcolepsy and in idiopathic hypersomnia.12 An increased body mass index has been noted in a few series.8,15
The overall psychosocial handicap of patients with idiopathic hypersomnia is similar to that of patients with narcolepsy.41
Diagnosis
Polysomnography
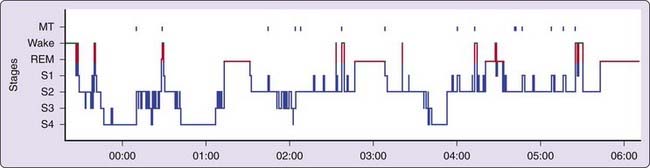
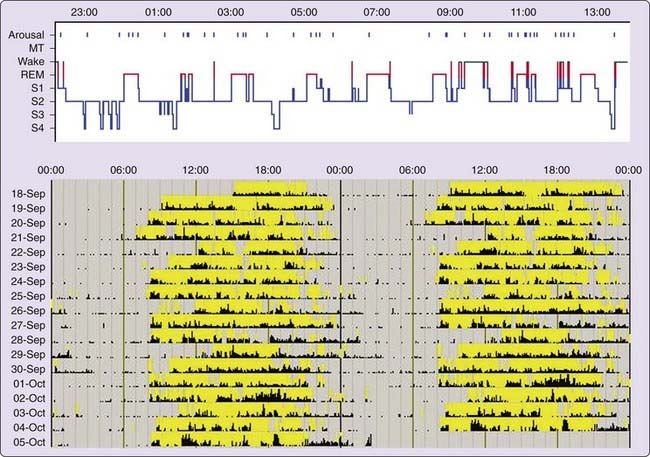
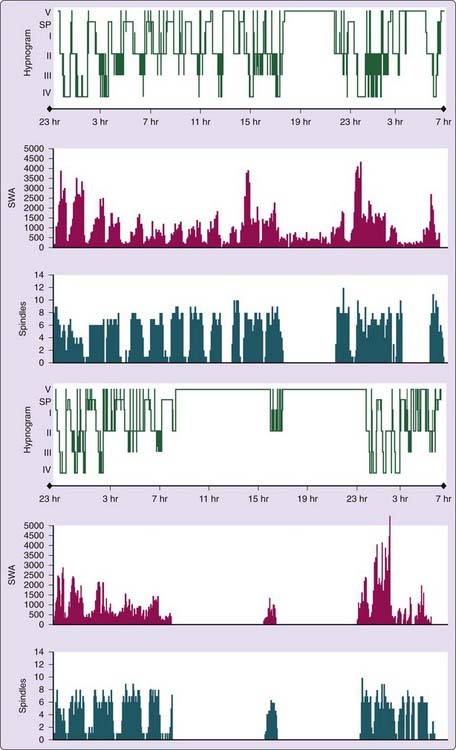
Polysomnographic findings of idiopathic hypersomnia include a short sleep latency, a high sleep efficiency (usually more than 90%), and increased amounts of deep (slow-wave) NREM sleep (Figs. 86-1 and 86-2).3,8,11,40 These findings are nonspecific and can be seen also in patients with BIISS (see later). Amounts of sleep spindles (throughout the sleep period or at the beginning and end of the night) have been occasionally reported to be elevated in idiopathic hypersomnia (Fig. 86-3).9,42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree