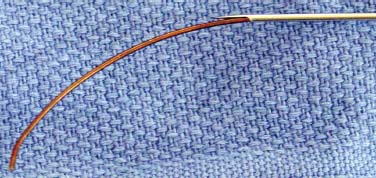
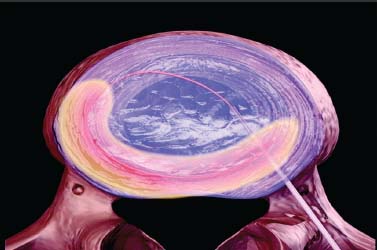
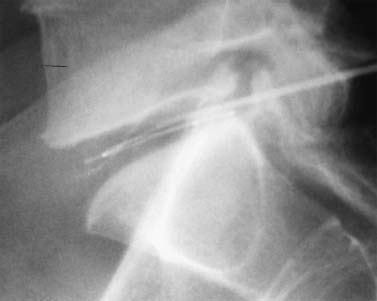
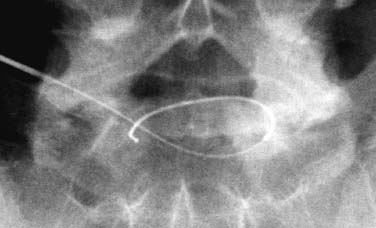
Until recently, treatment for predominant axial back pain caused by discogenic disease has consisted of a variety of noninvasive measures. These interventions encompass oral analgesics, oral anti-inflammatory agents, physical therapy, and epidural space steroid instillation. With these interventions, some patients experience relief, but many do not. A portion of those with intractable back pain are candidates for more aggressive measures and, in particular, a lumbar fusion. Prior to the advent of intradiskal electrothermal annuloplasty, there was no intervening therapeutic tool between rehabilitation and fusion. This new procedure involves placing a navigable electrothermal catheter within a symptomatic lumbar disk. Thermal energy is then conducted into the posterior annular wall during a timed protocol. The heating of the annular wall is theorized to cause coagulation and collagen denaturation that may result in a stabilizing effect to the disk.1,2 Ablation of nociceptors within the disk by thermal treatment has also been postulated.3 The U.S. Food and Drug Administration approved its use in 1998, and over 30,000 procedures have been performed in the United States to date. Since its inception, several outcome studies have been performed with varying results. Karasek and Bogduk4 reported on the 1-year outcome of 35 patients treated with intradiskal electrothermal therapy (IDET) and compared them with a control group of 17 patients similarly diagnosed but denied insurance authorization for IDET. Although both groups received similar rehabilitation measures, suggesting no treatment bias, the same conclusion is not applicable when considering the inclusion criteria. It must be emphasized that there was an inherent bias in the control group because they were denied this new and potentially conclusive procedure. Sixty percent of the treatment group experienced satisfactory results, which were defined as at least 50% reduction of their preprocedure visual analogue scale (VAS) rating, return to work, and a final VAS score of less than 4. Of the entire group receiving this intervention, 23% of the patients had complete relief of pain. Correcting for the relatively small sample size, this complete pain-free success rate of 23% carries a 95% confidence interval of ±14%. Thus, were this study to be repeated, complete relief could be observed in as much as 37% or as little as 9% of patients. The 95% confidence interval for the satisfactory group resulted in a range of 44 to 76%. Only one patient in the control group improved, whereas the remainder continued to have their preprocedure pain intensity. Bogduk and Karasek5 reported on this same population with 2-year follow-up documenting stable and enduring outcomes. Satisfactory success rates at 2 years marginally decreased to 54%, and complete relief of pain was observed in 20% of patients. Derby et a16 reported that 62.5% of IDET-treated patients had a favorable outcome, defined as improvement in three of the following four outcome tools: the Roland-Morris Low Back Pain and Disability Questionnaire (RM), VAS, the North American Spine Society Low Back Pain Outcome Assessment Instrument Patient Satisfaction Index (PSI), and a general activities of daily living questionnaire (ADL) modified from the same instrument. Twenty-five percent of patients showed no change at 12 months, whereas 12.5% patients had an unfavorable outcome, defined as worsening of three of the four outcome scales. Although a 62.5% favorable outcome is significant, the mean improvement in individual scores is unimpressive and may not be clinically significant. The mean decrease in the VAS was 1.84 (SD = 2.38) and in the RM was 4.03 (SD = 4.82). With respect to specific activities, 41% were improved in sitting, 50% in standing, 45% in walking, and 41% in sleeping, although the magnitude of improvement was not quantified. An interesting aspect of this study is the conclusions that can be drawn concerning technique. Seventythree percent of the IDET-treated patients experienced a favorable outcome if the active catheter tip incorporated 75 to 100% of the posterior annular wall. In contrast, 16.7% of the treated patients had a favorable outcome when less than 50% of the posterior annular wall was treated. Saal and Saal reported on the outcome of patients treated with IDET at a minimum of 6 months,7 12 months,8 and 2 years9 follow-up. In the latter study,9 consisting of 58 patients, a statistically and clinically significant improvement in VAS and bodily pain scores on the Medical Outcomes Study Short-Form General Health Survey (SF-36) was observed. The study group demonstrated a clinically significant improvement in physical function as supported by statistically significant improvement in sitting tolerance and physical function SF-36 scores. The conclusion that these results were clinically significant stem from two studies that have validated the SF-36 as a reliable instrument. Patrick et al10 had reported that a change in score of 17.7 on the physical function scale or 21.5 on the bodily pain scale is clinically significant. Subsequently, Deyo et al11 demonstrated that a 7-point change on any of the subscales is clinically significant. Consequently, the outcome study by Saal and Saal, in which a change in score of 31.33 on physical function and of 21.86 on bodily pain were reported, is indeed clinically significant. In a prospective nonrandomized clinical trial involving 27 consecutive patients with a follow-up of 1 year, Gerszten et al12 noted that 75% of them improved based on the Oswestry low back pain disability questionnaire. Only 48% of patients were found to improve according to the SF-36 survey. There was no relationship identified between outcome and duration of symptoms (p = .32), number of levels treated (p = .20), or workers’ compensation (p = .38). In a prospective case series consisting of 33 patients with a mean follow-up of 15 months, Lutz et al13 demonstrated a mean change in the VAS score of 3.9 (p < .001), a mean change in the lower extremity VAS score of 3.7 (p < .001), and a mean change in the RM of 7.3 (p < .001). Seventy-five percent of patients reported that they would undergo the same procedure for the same outcome. Complete pain relief was achieved in 24% of the patients and partial pain relief in 46% of the patients. The exact mechanism by which IDET produces its reported clinical effects remains unknown. Kleinstuck et al14 attempted to study intradiskal temperature dispersion from the SpineCATH. They were able to measure temperatures of greater than 42°C (temperature sufficient to thermocoagulate unmyelinated nerve fibers) at distances greater than 10 mm from the probe. It has been suggested that their use of previously frozen cadaveric disks and the placement of the heating elements in the nuclear cavity rather than in the anulus, as is done in clinical practice, may have limited the peak temperatures.9 There is a dearth of peerreviewed published literature assessing in vivo temperature dispersion from the SpineCath and the hypothesized resultant protein denaturation. In a recent report, Shah et al1 found microscopic evidence of acute collagen modulation in cadaveric disks heated with a SpineCATH. In an earlier cadaveric biomechanical study, Lee et al2 did not note any change in stability of the lumbar spine before and after treatment with IDET. Long-term follow-up studies need to be performed to delineate the biomechanical consequences of denaturation of intradiskal collagen. Based on the above studies, and in particular the 2-year report by Bogduk and Karasek,5 IDET represents a minimally invasive option for a select group of patients with chronic discogenic pain, proven by nonsedated provocative diskography, who have failed to improve with a comprehensive, exercise-based rehabilitation care program and who desire functional improvement but are reticent to undergo a lumbar fusion. Indications The IDET procedure is indicated in patients with low back pain caused by internal disk disruption syndrome. The inclusion criteria consist of function-limiting low back pain of at least 6 months’ continuous duration and failure to improve from an appropriate nonsurgical, conservative rehabilitation program of at least 4 months’ duration. In our view, an appropriate regimen comprises progressive intensive exercise, at least two fluoroscopically guided epidural space corticosteroid injections, a trial of manual therapy (provided it is early in the course), oral anti-inflammatory medication, nonnarcotic analgesics, and activity modification. An MRI study should demonstrate a degenerative disk without a focal protrusion or any evidence of neural compression at the proposed IDET disk level. A concordant pain response must be obtained with provocative diskography at low pressurization at one or more disk levels with adjacent control levels not demonstrating pain reproduction.5,7,8 Contraindications Contraindications to the IDET procedure include systemic infection, osteomyelitis, diskitis, cellulitis, complete collapse of the disk space, the presence of sequestered or extruded disk herniations, cauda equina syndrome, gross instability, and uncorrectable bleeding diathesis. The presence of hardware previously used for a lumbar fusion or a spinal cord stimulator theoretically precludes the performance of IDET. Performing IDET under these latter circumstances could result in inadvertent heating of nondiskal structures, so extreme caution is suggested. Moderately degenerated disks represent a relative contraindication. Such disks create a technical challenge, and even when the procedure is performed properly, the outcome may be disappointing. Instruments and Preparations Instruments The following instruments are essential: ORA-50 S Electrothermal Spine generator, single-use SpineCATH intradiskal electrothermal catheter, 17-gauge introducer needle, connector cable from the spine generator to the electrothermal catheter, fluoroscopy machine, standard sterile prep kit, sterile towels, sponge clamp, four 3 cc syringes, one 6-inch, 25-gauge needle, sterile gloves, and blood pressure and pulse oximeter. The SpineCath catheter is a flexible device with a curved tip (Fig. 31–1). Anesthesia Intravenous access is obtained prior to the procedure, and light conscious sedation is given. We have found the combination of Versed and fentanyl to work extremely well in our patients. For most patients, a starting dose of fentanyl of 75 µg is typically sufficient, provided the introducer needle and catheter are placed expeditiously. When difficulty is encountered, another 25 to 50 µg may be required. One milligram of Versed is appropriate for most patients. Of course, the doses must be modified according to patient size, debility, narcotic use history, and degree of anxiety. Heavy sedation should be avoided secondary to its potential cardiac and pulmonary complications. In addition, the patient needs to be responsive during the procedure to communicate any sudden onset of pain, dysesthesias, or paresthesias during advancement of the introducer needle and during the heating protocol. If the patient is not alert enough to recognize or inform the physician of such symptoms, then permanent neural injury may transpire. FIGURE 31–1 IDET catheter revealing curved tip. Positioning The positioning is physician-dependent and relates to the surgeon’s usual diskography technique. The patient is placed in the prone or prone oblique position on the fluoroscopy table. An extraforaminal approach is used, because IDET should never be performed via transdural placement. Surgical Technique A standard diskography technique is used to gain access to the disk. This involves securing an oblique view of the lumbar spine in which the superior articular process (SAP) of the vertebral body crosses the intervertebral disk and divides it into half posterior to the SAP and half anterior to the SAP for the L4–L5 and L5–S1 disk. Lumbar disks cephalad to the L4–L5 level should have the SAP bisect the disk in a 60:40, rather than 50:50, proportion. In the oblique view, the superior and inferior vertebral end plates should be aligned in parallel to allow the introducer needle to be placed in the midportion of the disk. Such placement enhances the operator’s ability to slink the catheter within the disk and not abut the end plates. The low back area is then prepped and draped in the usual sterile manner. Using a sponge clamp for a marker, the subcutaneous skin and tissues are anesthetized with 1% lidocaine. The 17-gauge introducer needle is then advanced to the posterolateral border of the disk. To avoid injuring the exiting nerve root, the introducer needle should remain just ventral to the SAP. Again, the introducer needle should enter the disk midway between the end plates to avoid injury to these sensitive structures. Once proper positioning is achieved, the needle is advanced through the posterolateral anulus. The needle tip is placed near the central nucleus, as confirmed in two planes by fluoroscopy. At this juncture, the needle should be aimed in a trajectory that would result in the catheter reaching the ventral and contralateral corner of the disk (approximately 10 o’clock if the middorsal disk represents 6 o’clock). Such placement is essential because the catheter will need to deflect off this “corner” and curve back toward the dorsal aspect of the disk. Intradiskal catheter function is then verified prior to placing it into the introducer needle. This is accomplished by attaching the connector cable to the generator and catheter. At this point, the generator should have been set in AutoTemp mode. Once connected, the actual temperature gauge should display room temperature. The impedance gauge should read within normal limits (85–230 ohms). If the impedence exceeds this amount, the catheter must be replaced because it is defective. Following the impedance check, the cable is detached from the catheter. The intradiskal catheter is inserted and advanced through the introducer needle until a change of resistance is appreciated, at which point the catheter has just exited the introducer needle and is now within the substance of the nucleus. It is then navigated intradiskally while viewing in the lateral plane. Gentle advancement and real-time fluoroscopic imaging of the catheter are strongly advised because it is easily bent and can crack. If a crack develops, the catheter will no longer be able to perform its heating task. In some instances, the catheter will circumnavigate the disk without difficulty. In other cases, the catheter may move superiorly or inferiorly and bounce off the end plate. When that occurs, the catheter must be withdrawn and advanced again using a different orientation. Reorientation of the catheter must always be accomplished in synchrony with the introducer needle to avoid damaging the catheter (Fig. 31–2). Remember that the introducer needle has a sharp beveled edge. Attempting to move the catheter in a manner that deposits the catheter against this edge with any degree of force can result in shearing of the catheter. There are times when the catheter is advanced, and the tip just sinks into the eroded anteroinferior margin of the disk. Again, the catheter must be reoriented before another attempt is made. Immediate reorientation is emphasized because there are only a limited number of passes that can be attempted before the catheter becomes unusable. Repeated unsuccessful attempts in which the catheter cannot be advanced without substantial force will result in fatigue and then fracture. If the catheter advances beyond an imaginary line connecting the most dorsal aspect of the vertebral bodies above and below the disk being treated, then the catheter must be withdrawn. At no point should the catheter rest in a position that could result in inadvertent heating of the neural elements in the epidural space. Similarly, the catheter should not enter or rest within the neural foramen. FIGURE 31–2 IDET catheter advanced through introducer needle, demonstrating development of a curve in the direction of the curved catheter tip and away from the sharp edge of the introducer needle. As the catheter is advanced in the lateral plane and curves around the anulus, it can pierce its outer border and enter the foramen. During advancement of the catheter the objective is to lodge the active portion adjacent to the inner anulus and across the entire posterior anulus. In other words, ideal placement involves covering the anulus from 3 to 9 o’clock (Fig. 31–3). This recommendation stems from our experience and two peer-reviewed papers. Derby et al6 demonstrated superior outcomes when a greater portion of the posterior anulus is covered. Slipman et al15 demonstrated that the annular tear in and of itself is not the painful structure, but an associated factor. Therefore, the goal is not “to cover the tear,” as some have insisted. Confirmation of proper positioning is aided by the presence of radiopaque markers on the catheter, which define the borders of the heating elements. FIGURE 31–3 Schematic diagram showing placement of the catheter from 3 to 9 o’clock position, covering almost 100% of posterior anulus. Once the catheter is appropriately placed, attention is then directed to the radiofrequency generator (Fig. 31–4). The set power gauge should display P90, which sets the peak temperature of the catheter tip. Standard protocol peak temperature is 90°C. The generator is then turned on by pressing the RF button once. As the heating process unfolds, the generator will automatically incrementally raise the set temperature by 1° every 30 seconds until the peak temperature is reached. At peak temperature, heating ensues for 4 minutes until the generator automatically discontinues energy delivery. Heating will transpire over a 16.5-minute duration provided the patient does not experience any clinically significant symptoms. If the patient describes leg pain, intolerable back pain, lower extremity paresthesias or dysesthesias, or involuntary leg contractions, then an alteration in the heating scheme must be initiated. Energy delivery can be manually discontinued by pressing and releasing either the foot pedal or the RF button at any time. If severe back pain is experienced, the heating protocol can be delayed, enabling the delivery of additional fentanyl or other analgesic. It is common for patients to experience some pain, frequently their usual discomfort, but it should be tolerable. Lower extremity symptoms other than mild diffuse discomfort automatically raise the concern of accidental neural injury. Heating must be discontinued and the placement of the catheter rechecked. Once heating is completed, the cable is disconnected from the catheter. The catheter is subsequently removed from the introducer needle while viewing under fluoroscopy. Hasty withdrawal could result in shearing of this very malleable catheter. Some advocate the administration of an intradiskal dose of antibiotic prior to removal of the introducer needle. Our preference is to avoid intradiskal placement of antibiotics. Instead, we routinely administer a dose of antibiotic through the IV 1 hour prior to the procedure. The introducer needle is then removed, and a 4 × 4 pressure dressing is placed over the insertion site. FIGURE 31–4 Electrothermal generator showing set power. Postoperative Care The patient is observed for 1 hour after the procedure for any complications and to allow the conscious sedation time to resolve. The IV is then removed, and the patient dons a soft lumbar orthosis and is discharged to home with an appointment to follow-up in the office in 2 weeks. The patient is instructed to wear the orthosis continuously over the initial 6-week postoperative interval, removing it only to shower or change clothes. The orthosis primarily serves as a reminder for the patient to limit his or her activities. We usually advise the patient to avoid all activities and rest for the first 3 days postop. After 3 days, the patient may begin walking short distances. Sitting is limited to 1-hour intervals with at least a 20-minute break between episodes for the first 2 weeks, then patients may increase their sitting time to tolerance. If they have a sedentary job, they may return to work as early as day 14. If their job entails heavy lifting, return to work is not recommended until 3 to 4 months postprocedure. Driving and light housework duties are allowed after 3 weeks. We typically restrict lifting to 0 to 10 lbs for the first 2 weeks, 10 to 25 lbs for 4 weeks, 25 to 50 lbs for 8 weeks, and then advance as tolerated. No bending or twisting activities are allowed through the first 6 weeks, at which time a graded flexibility and strengthening rehabilitation program is instituted. Progression to more advanced rehabilitation measures depends on the ultimate goal of each patient and is therefore individualized. Complications Intraoperative/Perioperative The spectrum and incidence of potential intraoperative complications of the IDET procedure are relatively small. While advancing the introducer needle past the SAP, one could pierce the traversing nerve root. Dural puncture, annular injury, and vertebral end plate injury are also complications that may occur while advancing the introducer needle into the disk. If the heating catheter migrates into the spinal canal, there exists the potential for cauda equina syndrome secondary to thermal injury. To date, there has been one reported case of cauda equina due to IDET.16 Uncontrolled bleeding and infection are potential complications. There has been one literature report of vertebral osteonecrosis associated with IDET, although the mechanism by which this complication occurred is unknown.17 Adverse reactions to anesthetic, conscious sedation medications, and antibiotic prophylaxis are also potential complications. Poor intravenous fluid management can result in peripheral edema and heart failure in patients at risk. Postoperative Diskitis, osteomyelitis, and epidural abscess are the primary infection-related complications. Nuclear herniation through the introducer needle diskal insertion site is a theoretical complication; however, there have been no such reported cases. Most patients experience an increase in their low back pain for approximately the first week following the procedure. Case Illustration A 32-year-old patient described back pain without leg pain that escalated over a 9-month interval. Although he had prior episodes of back pain during the past 6 years, each episode was limited to no more than 6 weeks’ duration. Between recurrences he experienced baseline low back pain that he rated 30 to 40 on a 100 mm VAS. During this recurrence, he could not perform his usual sedentary activities because of impaired concentration resulting from continued pain and because of a limited sitting tolerance. He participated in active therapy with experienced physical therapists, used oral anti-inflammatory agents, and underwent three epidural space steroid instillations. Despite these efforts, his symptoms were not ameliorated. At that juncture, he was prepared to undergo IDET or a fusion procedure provided it was indicated. A nonsedated provocative diskogram demonstrated the level of involvement to be L5–S1. IDET was then performed (Figs. 31–5, 31–6). At 1 month postop there was 90% symptom reduction, which progressed to 98% at 2 months. Thereafter the symptoms remained at this level, less than 5 on a 100 mm scale, for the ensuing 2 years. FIGURE 31–5 Lateral view of the introducer needle and catheter within the L5–S1 disk space. Intradiskal electrothermal therapy IDET is a minimally invasive procedure for treatment of patients with a diagnosis of lumbar internal disk disruption syndrome. Peer-reviewed publications have demonstrated that it is a safe procedure with minimal adverse events. Its advantages in the treatment of discogenic pain as compared with open surgery reside in its ability to minimize tissue destruction, decrease hospital expenses, reduce potential complications, and allow for quicker recovery time. Although the outcome studies that have been published suggest this is a worthwhile intervention for patients with internal disk disruption, the results are not overwhelming. Perhaps further inquiry will identify the inclusion and exclusion criteria that will afford higher success rates in a constant and predictable manner. FIGURE 31–6 Posteroanterior view of the introducer and needle within the L5–S1 disk. REFERENCES
31

Intradiskal Electrothermal Therapy



< div class='tao-gold-member'>
Intradiskal Electrothermal Therapy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








